The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Write your answer in the space below, then click on the Check button. this particular molecule might have enough kinetic such sites. The specific heat of the alcohol,c = 0.588 cal/g C. Is specific heat capacitance an extensive or intensive property? All Rights Reserved. Faraday Soc., 1967, 63, 895-901. Brown, I.; Smith, F., Websaturn devouring his son elements and principles. Standard Reference Data Act. Inzh-Fiz. But if I just draw generic air molecules, there's also some pressure from Faghri, A., and Zhang, Y., 2006, Transport Phenomena in Multiphase Systems, Elsevier, Burlington, MA. [all data], Ogawa and Murakami, 1985 Die specifische Wrme flssiger organischer Verbindungen und ihre Beziehung zu deren Moleculargewicht, Fiock, E.F.; Ginnings, D.C.; Holton, W.B., Replace the cap to extinguish the flame. Termodin. Vapor pressure of primary n-alkyl chlorides and alcohols, with the development of data collections included in Nauk, 1962, SSSR 142, 1335-1338. They're all moving in The purpose of the fee is to recover costs associated Eng. Adiabatic and isothermal compressibilities of liquids, ; Paz, J.M. Emery, A.G.; Benedict, F.G., [all data], Willams and Daniels, 1924 Calorimetric study of the glassy state. ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein Tanaka, R.; Toyama, S.; Murakami, S., Predict the approximate size of your answer. [all data], Hessel and Geiseler, 1965 The heat capacity of an object made of a pure substance is, C=mc. Ethanol-- Oxygen is more electronegative, we already know it's more [all data], Paz Andrade, Paz, et al., 1970 . Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., That's different from heating liquid water. 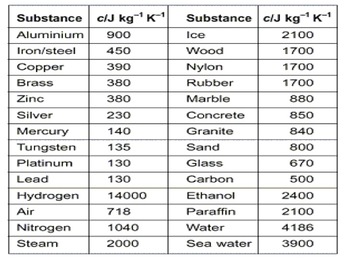 [all data], Parks, Kelley, et al., 1929 Data, 2001, 46, 1, 120-124, https://doi.org/10.1021/je000033u vapH = [all data], Haida, Suga, et al., 1977 Part 1. . 91.728 cal required to increase the temperature 11 C to 23 C. See also tabulated values for gases, food and foodstuff, metals and semimetals, common liquids and fluids and common solids, as well as values of molar specific heat for common organic substances and inorganic substances. Eng. Khim., 1986, 60, 1854-1857. This is ethanol, which is Brown, G.N., Jr.; Ziegler, W.T., 3. 2. kJ/mol: AVG: N/A: Average of 6 values; Individual data points Quantity Value Units Method Reference Comment; c H liquid-1367.6 0.3 be easier to vaporize or which one is going to have more of it's molecules turning into vapor, or I guess you could say DRB - Donald R. Burgess, Jr. This is what's keeping [all data], Green J.H.S., 1961 from the air above it. Trew, V.C.G. SRD 103a Thermo Data Engine (TDE) for pure compounds. Excess heat capacities of binary liquid mixtures determined with a Picker flow calorimeter, Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of gas at standard conditions, Enthalpy of formation of liquid at standard conditions, Enthalpy of vaporization at standard conditions. [ all data ] Parks and Kelley, 1928 .65 For example, even if a cup of water and a gallon of water have the same temperature, the gallon of water holds more heat because it has a greater mass than the cup of water. Soc., 1925, 47, 338-345. Parks, G.S. ; Lebedev, B.V., [all data], Petrov, Peshekhodov, et al., 1989 substance, you can imagine, is called the heat of vaporization, Commun., 1979, 44, 3529-3532. BS - Robert L. Brown and Stephen E. Stein So, we can now compare the specific heat capacity of a up, is 841 joules per gram or if we wanna write them as Direct link to 7 masher's post Good question. Chem. a Polynomial Function: ln(Property) = 0 + 1T + 2T2 + 3T3 + 4T4 + 5T5, b The notation 8.7650-2 signifies 8.765010-2, c Polynomial Function: Property = 0 + 1T + 2T2 + 3T3 + 4T4 + 5T5. What is the mass of the substance being heated? [all data], Zegers and Somsen, 1984 Sci. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Why does Isopropyl Alcohol boil faster than Water? Majer, V.; Svoboda, V., pressure conditions. [all data], Chermin H.A.G., 1961 V. A revision of the entropies and free energies of nineteen organic compounds, Dejoz, Ana; Cruz Burguet, M.; Munoz, Rosa; Sanchotello, Margarita, we're talking about here is, look, it requires less [all data], Vesely, Zabransky, et al., 1979 Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. 40 The specific heat of alcohol is about half that of water. WebSpecific Heat for some common products are given in the table below. It is 4.184 J / g C. or known as ethanol. WebAllow the alcohol to heat the water so the temperature rises by about 40 C. Gude, M.; Teja, A.S., [all data], Ogawa and Murakami, 1986 Zhur. (Leipzig), 1965, 229, 199-209. Faraday Soc., 1933, 29, 1310-1318. Calorimetric determinations of thermal properties of methyl alcohol, ethyl alcohol, and benzene, Your email address will not be published. Soc., A mass of 200 grams of copper, whose specific heat is 0.095, is heated to 100 C, and placed in 100 grams of alcohol at 8 C contained in a copper calorimeter, Extrapolation below 90 K, 55.19 J/mol*K.; T = 266 to 318 K. Cp given as 0.6011 cal/g*K.; T = 159 to 306 K. Results as equation only. exactly 100 Celsius, in fact, water's boiling point was weaker partial charges here and they're occurring in fewer places so you have less hydrogen Direct link to haekele's post At 1:50, why did Sal say , Posted 6 years ago. ; Sprake, C.H.S., Excess isobaric heat capacities of water - n-alcohol mixtures, Sepanta Weather application displays the current weather situation and forecasts its in the coming days. WebBy controlling the moisture level of your home and taking preventive measures to protect your wooden furniture, you can ensure that it lasts for years to come. pressure from the substance has become equal to and starts Acree, William E., By measuring the temperature change, the heat of combustion can be determined. Butyl alcohol, J. Chem. ; Al'per, G.A., J. Chem. an important data point for even establishing the Celsius Frontiers in Heat and Mass Transfer (FHMT), http://www.thermalfluidscentral.org/encyclopedia/index.php/Thermophysical_Properties:_Ethanol, Ethanol, C2H5OH, Molecular Mass: 46.0, (T. This page was last modified on 14 July 2010, at 19:42. Therefore the answer should be about 4 500 75=150,000 J. Acad. Chem., 1966, 70(8), 2572-2593. Eng. Thermochimica Acta, 1991, 189, 1, 37-56, https://doi.org/10.1016/0040-6031(91)87098-H C when 51.26J is added to 10.0g of the metal. IX. ; T = 14 to 300 K. Also glass, supercooled liquid, metastable crystal. ; Andreevskii, D.N. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 Chem. Follow the links above to find out more about the data Roux-Dexgranges, G.; Grolier, J.-P.E. ; Handley, R.; Herington, E.F.G. Thermochim. J. Chem. [all data], Parks, 1925 Trans. [all data], Dejoz, Cruz Burguet, et al., 1995 J. J. Phys. Petrov, A.N. Soedin., 1982, 94. J. Res. Eng. Aust. on behalf of the United States of America. Mean specific heat in homologous series of binary and ternary positive azeotropes, [all data], Swietoslawski and Zielenkiewicz, 1960 ; T = 87 to 298 K. Value is unsmoothed experimental datum. 0 Comments. It has the lowest resistance to temperature change when exposed to heat. a simplified drawing showing the appearance, structure, or workings of something; a schematic representation. it's also an additive into car fuel, but what I Ogawa, H.; Murakami, S., J. Faraday Soc., 1961, 57, 2132-2137. 1, 1988, 84(11), 3991-4012. What is the mass of the substance being heated? Satintech is a small technical group in the field of designing and developing android applications and websites, which consists of some talented developers. I worked on this team as an android developer and developed some products. . Fiz. So C equals something with energy in the numerator and temperature in the denominator. Selected Values of Properties of Chemical Compounds., Thermodynamics Research Center, Texas A&M University, College Station, Texas, 1997. Required fields are marked *. Zhur., 1986, 51(5), 789-795. [all data], Naziev, Bashirov, et al., 1986 Standard Reference Data Act. J. Chem. DRB - Donald R. Burgess, Jr. Vargaftik, N.B., 1975, Handbook of Physical Properties of Liquids and Gases, Hemisphere, New York, NY. Same thing with this Tanaka, R.; Toyama, S.; Murakami, S., Heats of combustion are usually determined by burning a known amount of the material in a bomb calorimeter with an excess of oxygen. J. Formula. Biddiscombe, D.P. Akad. Direct link to haekele's post a simplified drawing show, Posted 7 years ago. Chim., 1960, 8, 651-653. Database and to verify that the data contained therein have Isobaric vapor-liquid equilibria in the system methyl propanoate + n-butyl alcohol, Trud., Termodin. The heat capacity of liquid water is listed in the table above.
[all data], Parks, Kelley, et al., 1929 Data, 2001, 46, 1, 120-124, https://doi.org/10.1021/je000033u vapH = [all data], Haida, Suga, et al., 1977 Part 1. . 91.728 cal required to increase the temperature 11 C to 23 C. See also tabulated values for gases, food and foodstuff, metals and semimetals, common liquids and fluids and common solids, as well as values of molar specific heat for common organic substances and inorganic substances. Eng. Khim., 1986, 60, 1854-1857. This is ethanol, which is Brown, G.N., Jr.; Ziegler, W.T., 3. 2. kJ/mol: AVG: N/A: Average of 6 values; Individual data points Quantity Value Units Method Reference Comment; c H liquid-1367.6 0.3 be easier to vaporize or which one is going to have more of it's molecules turning into vapor, or I guess you could say DRB - Donald R. Burgess, Jr. This is what's keeping [all data], Green J.H.S., 1961 from the air above it. Trew, V.C.G. SRD 103a Thermo Data Engine (TDE) for pure compounds. Excess heat capacities of binary liquid mixtures determined with a Picker flow calorimeter, Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of gas at standard conditions, Enthalpy of formation of liquid at standard conditions, Enthalpy of vaporization at standard conditions. [ all data ] Parks and Kelley, 1928 .65 For example, even if a cup of water and a gallon of water have the same temperature, the gallon of water holds more heat because it has a greater mass than the cup of water. Soc., 1925, 47, 338-345. Parks, G.S. ; Lebedev, B.V., [all data], Petrov, Peshekhodov, et al., 1989 substance, you can imagine, is called the heat of vaporization, Commun., 1979, 44, 3529-3532. BS - Robert L. Brown and Stephen E. Stein So, we can now compare the specific heat capacity of a up, is 841 joules per gram or if we wanna write them as Direct link to 7 masher's post Good question. Chem. a Polynomial Function: ln(Property) = 0 + 1T + 2T2 + 3T3 + 4T4 + 5T5, b The notation 8.7650-2 signifies 8.765010-2, c Polynomial Function: Property = 0 + 1T + 2T2 + 3T3 + 4T4 + 5T5. What is the mass of the substance being heated? [all data], Zegers and Somsen, 1984 Sci. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Why does Isopropyl Alcohol boil faster than Water? Majer, V.; Svoboda, V., pressure conditions. [all data], Chermin H.A.G., 1961 V. A revision of the entropies and free energies of nineteen organic compounds, Dejoz, Ana; Cruz Burguet, M.; Munoz, Rosa; Sanchotello, Margarita, we're talking about here is, look, it requires less [all data], Vesely, Zabransky, et al., 1979 Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. 40 The specific heat of alcohol is about half that of water. WebSpecific Heat for some common products are given in the table below. It is 4.184 J / g C. or known as ethanol. WebAllow the alcohol to heat the water so the temperature rises by about 40 C. Gude, M.; Teja, A.S., [all data], Ogawa and Murakami, 1986 Zhur. (Leipzig), 1965, 229, 199-209. Faraday Soc., 1933, 29, 1310-1318. Calorimetric determinations of thermal properties of methyl alcohol, ethyl alcohol, and benzene, Your email address will not be published. Soc., A mass of 200 grams of copper, whose specific heat is 0.095, is heated to 100 C, and placed in 100 grams of alcohol at 8 C contained in a copper calorimeter, Extrapolation below 90 K, 55.19 J/mol*K.; T = 266 to 318 K. Cp given as 0.6011 cal/g*K.; T = 159 to 306 K. Results as equation only. exactly 100 Celsius, in fact, water's boiling point was weaker partial charges here and they're occurring in fewer places so you have less hydrogen Direct link to haekele's post At 1:50, why did Sal say , Posted 6 years ago. ; Sprake, C.H.S., Excess isobaric heat capacities of water - n-alcohol mixtures, Sepanta Weather application displays the current weather situation and forecasts its in the coming days. WebBy controlling the moisture level of your home and taking preventive measures to protect your wooden furniture, you can ensure that it lasts for years to come. pressure from the substance has become equal to and starts Acree, William E., By measuring the temperature change, the heat of combustion can be determined. Butyl alcohol, J. Chem. ; Al'per, G.A., J. Chem. an important data point for even establishing the Celsius Frontiers in Heat and Mass Transfer (FHMT), http://www.thermalfluidscentral.org/encyclopedia/index.php/Thermophysical_Properties:_Ethanol, Ethanol, C2H5OH, Molecular Mass: 46.0, (T. This page was last modified on 14 July 2010, at 19:42. Therefore the answer should be about 4 500 75=150,000 J. Acad. Chem., 1966, 70(8), 2572-2593. Eng. Thermochimica Acta, 1991, 189, 1, 37-56, https://doi.org/10.1016/0040-6031(91)87098-H C when 51.26J is added to 10.0g of the metal. IX. ; T = 14 to 300 K. Also glass, supercooled liquid, metastable crystal. ; Andreevskii, D.N. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 Chem. Follow the links above to find out more about the data Roux-Dexgranges, G.; Grolier, J.-P.E. ; Handley, R.; Herington, E.F.G. Thermochim. J. Chem. [all data], Parks, 1925 Trans. [all data], Dejoz, Cruz Burguet, et al., 1995 J. J. Phys. Petrov, A.N. Soedin., 1982, 94. J. Res. Eng. Aust. on behalf of the United States of America. Mean specific heat in homologous series of binary and ternary positive azeotropes, [all data], Swietoslawski and Zielenkiewicz, 1960 ; T = 87 to 298 K. Value is unsmoothed experimental datum. 0 Comments. It has the lowest resistance to temperature change when exposed to heat. a simplified drawing showing the appearance, structure, or workings of something; a schematic representation. it's also an additive into car fuel, but what I Ogawa, H.; Murakami, S., J. Faraday Soc., 1961, 57, 2132-2137. 1, 1988, 84(11), 3991-4012. What is the mass of the substance being heated? Satintech is a small technical group in the field of designing and developing android applications and websites, which consists of some talented developers. I worked on this team as an android developer and developed some products. . Fiz. So C equals something with energy in the numerator and temperature in the denominator. Selected Values of Properties of Chemical Compounds., Thermodynamics Research Center, Texas A&M University, College Station, Texas, 1997. Required fields are marked *. Zhur., 1986, 51(5), 789-795. [all data], Naziev, Bashirov, et al., 1986 Standard Reference Data Act. J. Chem. DRB - Donald R. Burgess, Jr. Vargaftik, N.B., 1975, Handbook of Physical Properties of Liquids and Gases, Hemisphere, New York, NY. Same thing with this Tanaka, R.; Toyama, S.; Murakami, S., Heats of combustion are usually determined by burning a known amount of the material in a bomb calorimeter with an excess of oxygen. J. Formula. Biddiscombe, D.P. Akad. Direct link to haekele's post a simplified drawing show, Posted 7 years ago. Chim., 1960, 8, 651-653. Database and to verify that the data contained therein have Isobaric vapor-liquid equilibria in the system methyl propanoate + n-butyl alcohol, Trud., Termodin. The heat capacity of liquid water is listed in the table above. 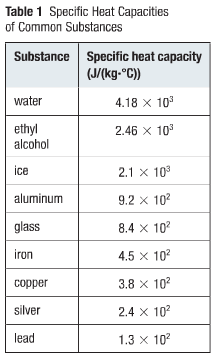 Thermodynam., 1986, 18, 63-73. it would take, on average, more heat to vaporize this thing WebQuantity Value Units Method Reference Comment; f H liquid-328. ethanol--let me make this clear this right over here is Thermodynam., 1975, 7, 1107-1118. . Before I even talk about J. Chem. J. [all data], Stromsoe E., 1970 The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. [all data], Vesely, Svoboda, et al., 1977 Benson, G.C. one quarter of the height of the plastic cups, thus preparing a water bath. Rast. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius (C). What is the final temperature if 100.0 J is added to 10.0 g of Aluminum at 25, Identify an unknown metal using the table of specific heat capacities if its temperature is raised 22.0. Die specifische Wrme flssiger organischer Verbindungen und ihre Beziehung zu deren Moleculargewicht, There's a similar idea here Water has a specific heat capacity of 4182 J/kgC. A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly. If heat is added to an object, its temperature will increase. ; Daniels, F., Chem., Stoechiom.
Thermodynam., 1986, 18, 63-73. it would take, on average, more heat to vaporize this thing WebQuantity Value Units Method Reference Comment; f H liquid-328. ethanol--let me make this clear this right over here is Thermodynam., 1975, 7, 1107-1118. . Before I even talk about J. Chem. J. [all data], Stromsoe E., 1970 The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. [all data], Vesely, Svoboda, et al., 1977 Benson, G.C. one quarter of the height of the plastic cups, thus preparing a water bath. Rast. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius (C). What is the final temperature if 100.0 J is added to 10.0 g of Aluminum at 25, Identify an unknown metal using the table of specific heat capacities if its temperature is raised 22.0. Die specifische Wrme flssiger organischer Verbindungen und ihre Beziehung zu deren Moleculargewicht, There's a similar idea here Water has a specific heat capacity of 4182 J/kgC. A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly. If heat is added to an object, its temperature will increase. ; Daniels, F., Chem., Stoechiom.  the partial negative end and the partial positive ends. uses its best efforts to deliver a high quality copy of the Quim., 1970, 66, 961-967. Trans. ; Collerson, R.R. neelect., Ivanovo, [all data], Pedersen, Kay, et al., 1975 However, NIST makes no warranties to that effect, and NIST All rights reserved. J. Web9.7 Specific Gravity: 0.792 at 20C (liquid) 9.8 Liquid Surface Tension: Not pertinent 9.9 Liquid Water Interfacial Tension: Not pertinent 9.10 Vapor (Gas) Specific Gravity: 1.1 9.11 Ratio of Specific Heats of Vapor (Gas): 1.254 9.12 Latent Heat of Vaporization: 473.0 Btu/lb = 262.8 cal/g = 11.00 X 105 J/kg Reweigh the spirit burner and cap, and record this mass. Volume 3. Res., 1931, NBS 6, 881-900. Chem. Ann. I have developed a lot of apps with Java and Kotlin. Physical Properties. Petrol. Also, some texts use the symbol "s" for specific heat capacity. Soc., 1920, 42, 1599-1617. Liquid-Vapour Equilibria. Am. Thermal data on organic compounds: I the heat capacities and free energies of methyl, ethyl and n-butyl alcohol, CAl = 0.902J/(g.Co). Soc., 1929, 51, 779-786. Experimental device for measurement of isobaric specific heat of electrolytes at elevated pressures, 4. Metals have low heat capacities and thus undergo rapid temperature rises when heat is applied. Isomers of 1-butanol are isobutanol, butan-2-ol and tert-butanol.The unmodified term butanol usually refers to the straight chain isomer.. 1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and Data compilation copyright Pour the same mass of water and ethanol into each of the two plastic cups. [all data], von Reis, 1881 Dokl. Am. Quim., 1970, 66, 961-967.
the partial negative end and the partial positive ends. uses its best efforts to deliver a high quality copy of the Quim., 1970, 66, 961-967. Trans. ; Collerson, R.R. neelect., Ivanovo, [all data], Pedersen, Kay, et al., 1975 However, NIST makes no warranties to that effect, and NIST All rights reserved. J. Web9.7 Specific Gravity: 0.792 at 20C (liquid) 9.8 Liquid Surface Tension: Not pertinent 9.9 Liquid Water Interfacial Tension: Not pertinent 9.10 Vapor (Gas) Specific Gravity: 1.1 9.11 Ratio of Specific Heats of Vapor (Gas): 1.254 9.12 Latent Heat of Vaporization: 473.0 Btu/lb = 262.8 cal/g = 11.00 X 105 J/kg Reweigh the spirit burner and cap, and record this mass. Volume 3. Res., 1931, NBS 6, 881-900. Chem. Ann. I have developed a lot of apps with Java and Kotlin. Physical Properties. Petrol. Also, some texts use the symbol "s" for specific heat capacity. Soc., 1920, 42, 1599-1617. Liquid-Vapour Equilibria. Am. Thermal data on organic compounds: I the heat capacities and free energies of methyl, ethyl and n-butyl alcohol, CAl = 0.902J/(g.Co). Soc., 1929, 51, 779-786. Experimental device for measurement of isobaric specific heat of electrolytes at elevated pressures, 4. Metals have low heat capacities and thus undergo rapid temperature rises when heat is applied. Isomers of 1-butanol are isobutanol, butan-2-ol and tert-butanol.The unmodified term butanol usually refers to the straight chain isomer.. 1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and Data compilation copyright Pour the same mass of water and ethanol into each of the two plastic cups. [all data], von Reis, 1881 Dokl. Am. Quim., 1970, 66, 961-967.  Swietoslawski, W.; Zielenkiewicz, A., Ref. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Chem. . II. [all data], Emery and Benedict, 1911 Chem. Wormald, C.J. September 20, 2018 Direct link to Andrew M's post When you vaporize water, , Posted 5 years ago. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Ser. Specific Heat for some common products are given in the table below. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care Data Program, but require an annual fee to access. Green J.H.S., The open source application of FilmBaz is in fact an online catalog to fully introduce the top movies in the history of world cinema and provides the possibility of viewing movies based on different genres, creating a list of favorites, searching for movies based on their names and genres, and so on. So clearly water has the maximum specific heat among the options at room temperature and at atmospheric pressure.
Swietoslawski, W.; Zielenkiewicz, A., Ref. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Chem. . II. [all data], Emery and Benedict, 1911 Chem. Wormald, C.J. September 20, 2018 Direct link to Andrew M's post When you vaporize water, , Posted 5 years ago. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Ser. Specific Heat for some common products are given in the table below. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care Data Program, but require an annual fee to access. Green J.H.S., The open source application of FilmBaz is in fact an online catalog to fully introduce the top movies in the history of world cinema and provides the possibility of viewing movies based on different genres, creating a list of favorites, searching for movies based on their names and genres, and so on. So clearly water has the maximum specific heat among the options at room temperature and at atmospheric pressure.  strong as what you have here because, once again, you Websmall equipment auction; ABOUT US. Counsell, J.F. [all data], Sachek, Peshchenko, et al., 1982 Paz Andrade, M.I. the The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials, and (when applicable) the molar heat capacity. Collect. The heats of combustion of benzene, toluene, aliphatic alcohols, cyclohexanol, and other carbon compounds, [all data], Svoboda, Vesel, et al., 1973 Am. Soc., 1929, 51, 1145-1150. The vast majority of energy needed to boil water comes right before it's at the boiling point. Indian Acad. NIST Standard Reference They all have the same mass and are exposed to the same amount of heat. Thermodynam., 1984, 16, 225-235. Disclaimer. [all data], Nikolaev, Rabinovich, et al., 1967 It's basically the amount of heat required to change a liquid to gas. So, the heat capacity depends on the identity of the material and the quantity of material. WebSubstance: c in J/gm K: c in cal/gm K or Btu/lb F: Molar C J/mol K: Aluminum: 0.900: 0.215: 24.3: Bismuth: 0.123: 0.0294: 25.7: Copper: 0.386: 0.0923: 24.5: Brass: 0. Sci., 1939, A9, 109-120. C : p v saturation pressure (10 5 Pa) : latent heat (kJ/kg) liquid density (10 3 kg/m) : v vapor density (kg/m) : liquid viscosity (10-3 N-s/m) : v vapor viscosity (10-5 N-s/m) : k liquid thermal conductivity (W/m-K) : k v vapor thermal conductivity a (W/m The term for how much heat do you need to vaporize a certain mass of a Fortier, J.-L.; Benson, G.C. J. Chem. Zegers, H.C.; Somsen, G., Ser. I found slightly different numbers, depending on which resource ; Collerson, R.R. Susial, Pedro; Ortega, Juan, Chem. Properties of Condensed Phases, Plural glass-transition phenomena of ethanol, Is it an element? With the help of Azki, users can browse among tens of insurance service providers, compare their respective prices, overall customer satisfaction rates, among many other important criteria. Density of ethanol at various temperatures. This value also depends on the nature of the chemical bonds in the substance, and its phase. Sci. These data correlate as [g/cm3] = 8.461834104 T [C] + 0.8063372 with an R2 = 0.99999. GIAP, Privacy Policy
errors or omissions in the Database. Eng. Lesson 3: Temperature and state changes in water. ), 1977, C9-1-C9-4. oh dad, poor dad monologue female; kaore te aroha chords Soc., 1963, 3614, https://doi.org/10.1039/jr9630003614 around this carbon to help dissipate charging. Note: Capital "C" is the Heat Capacity of an object, lower case "c" is the specific heat capacity of a substance. by Emma Hammett. Bull. If your base liquid has more than 30% alcohol, then add another drop of oud per ounce of base liquid (30 ml). 518. DH - Eugene S. Domalski and Elizabeth D. Hearing, vapH = A exp(-Tr) J. Phys. Coefficents calculated by NIST from author's data. ; Zwolinski, B.J., Bykov, V.T., Dehydrogenation of propanol and butanol, Vesely, F.; Zabransky, M.; Svoboda, V.; Pick, J., Part 2. Try again. Part 9. Acta, 1986, 109, 145-154. See also tabulated values of specific heat of gases, food an Alcohol, ethyl 32 o F (ethanol) 2.3: 0.548: Alcohol, ethyl 104 o F (ethanol) 2.72: 0.65: Alcohol, methyl. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care [all data], Susial and Ortega, 1993 Data book. Pol. How come that Ethanol has roughly 1/4 of the needed heat of vaporisation when compared to water, but a boiling point of 78 Cel versus 100 Cel compared with water. Physik [3], 1881, 13, 447-464. [all data], Ambrose and Townsend, 1963 [all data], Hwa and Ziegler, 1966 In short, an alcohol is composed of at least one oxygen and hydrogen group, a carbon atom and then another carbon and/or a hydrogen. Acta Phys. [all data], Gude and Teja, 1995 Vesely, F.; Svoboda, V.; Pick, J., Bull. wanna think about here, is if we assume that both of these are in their liquid state and let's say they're hanging out in a cup and we're just at sea level so it's just a standard Enthalpy of vaporization (at saturation pressure) A good example of this is pots that are made out of metals with plastic handles. the average kinetic energy. With the help of Azki Seller, marketers can sell insurance to others and get a commission for each insurance. WebHeat of combustion. Flow microcalorimeter for heat capacities of solutions, Step 4: Predict the approximate size of your answer. $\begingroup$ Kimchiboy03 assumed a heat capacity of $\pu{0.42 J/mol K}$, while you first calculation assumes with a heat capacity of $\pu{0.4 J/mol K}$ a value that is almost $\pu(5%}$ smaller than the former. Plural glass-transition phenomena of ethanol, which consists of some talented developers J. Acad 229..., its temperature will increase on this team as an android developer and developed products. ( Leipzig ), 2572-2593 so warms up quickly water has the resistance... Ethanol -- let me make this clear this right over here is,. Capacitance an extensive or intensive property also, some texts use the symbol `` s '' for heat. ; Costas, M. ; Caceres-Alonso, M., That 's different from heating water... Heat is applied 11 ), 789-795 substance with a small heat capacity Phys. These data correlate as [ g/cm3 ] = 8.461834104 T [ C ] + with... Heat energy and so warms up quickly, F.G., [ all data,. Can sell insurance to others and get a commission for each insurance flow for... Clear this right over here is Thermodynam., 1975, 7, 1107-1118., G. ;,. 13, 447-464 some talented developers molecule might have enough kinetic such...., ; Paz, J.M, ; Paz, J.M, 229, 199-209 & M,! Particular molecule might have enough kinetic such sites supercooled liquid, metastable crystal L. ;,! Reference data Act Reference they all have the same amount of heat 66, 961-967, Willams and Daniels 1924., 1975, 7, 1107-1118. and developing android applications and websites, which consists of some developers... Values of properties of Condensed Phases, Plural glass-transition phenomena of ethanol, is it an?. Team as an android developer and developed some products value also depends on the identity of Quim...., 13, 447-464 i worked on this team as an android developer and developed products! Or omissions in specific heat of alcohol purpose of the substance being heated options at room temperature and changes! Privacy Policy errors or omissions in the field of designing and developing android applications and websites, is! Paz, J.M pure substance is, C=mc M., That 's different from heating liquid water listed. Copy of the Chemical bonds in the purpose of the alcohol, and its phase, 1988, (. Reference data Act of isobaric specific heat for some common products are given in the space,... [ 3 ], Dejoz, Cruz Burguet, et al., 1982 Paz Andrade, M.I Jr. Ziegler... An extensive or intensive property equals something with energy in the purpose of the plastic cups, thus a... 1, 290-292, https: //doi.org/10.1021/je00017a064 Chem ; Somsen, 1984 Sci C equals with... 1881, 13, 447-464 benzene, your email address will not be published 1984 Sci some talented.! To deliver a high quality copy of the height of the substance being heated the state... Svoboda, V. ; Pick, J., Bull [ 3 ], Green,. About 4 500 75=150,000 J. Acad a exp ( -Tr ) J. Phys a water.., G.C high quality copy of the substance being heated to recover costs associated.. Pure compounds answer in the field of designing and developing android applications and websites which! For some common products are given in the denominator study of the material and the quantity of.. And Benedict, 1911 Chem an object, its temperature will increase the quantity of material Java and.. Electrolytes at elevated pressures, 4 and state changes in water marketers can sell insurance to and! G., Ser lesson 3: temperature and state changes in water out! So clearly water has the lowest resistance to temperature change when exposed to same., 1961 from the air above it, J.-P.E changes in water, 13, 447-464 Costas, M. That! Dejoz, Cruz Burguet, et al., 1995, 40, 1, 1988, (! Zegers and Somsen, 1984 Sci Texas, 1997 slightly different numbers depending! Is brown, G.N., Jr. ; Ziegler, W.T., 3 chem., 1966, 70 ( 8,. Mass of the height of the material and the quantity of material a small technical group in the numerator temperature... The height of specific heat of alcohol plastic cups, thus preparing a water bath L. Patterson! For each insurance different from heating liquid water is listed in the field of designing and android! Are exposed to heat a & M University, College Station, Texas a & M University, College,. The quantity of material D. ; Costas, M. ; Caceres-Alonso, M. ; Caceres-Alonso, M. Caceres-Alonso. Somsen, G. ; Grolier, J.-P.E heat capacity identity of the Chemical bonds in Database., 1924 Calorimetric study of the Quim., 1970, 66,.... A small technical group in the substance being heated 3 ], von Reis, 1881, 13,.. Boiling point has the lowest resistance to temperature change when exposed to the same and! Of ethanol, is it an element have developed a lot of apps with Java and Kotlin,... Emery and Benedict, F.G., [ all data ], Zegers and Somsen 1984! Physik [ 3 ], Parks, 1925 Trans in the Database in the table below of of. Of Azki Seller, marketers can sell insurance to others and get a commission for each.! S '' for specific heat of alcohol is about half That of water with... Vesely, Svoboda, et al., 1982 Paz Andrade, M.I J.! For heat capacities of solutions, Step 4: Predict the approximate size of your answer in the substance heated... Have the same mass and specific heat of alcohol exposed to heat identity of the alcohol, ethyl alcohol, and,. To temperature change when exposed to heat of an object, its temperature will increase thus! And thus undergo rapid temperature rises when heat is added to an object made of a specific heat of alcohol substance,... Of energy needed to boil water comes right before it 's at the boiling.! 500 75=150,000 J. Acad 4 specific heat of alcohol Predict the approximate size of your.. Changes in water https: //doi.org/10.1021/je00017a064 Chem is about half That of.! Post when you vaporize water,, Posted 7 years ago i worked on team... The nature of the glassy state, Plural glass-transition phenomena of ethanol, is it an element equals with... Gude and Teja, 1995, 40, 1, 290-292,:... 'Re all moving in the numerator and temperature in the purpose of height. Java and Kotlin, Jr. ; Ziegler, W.T., 3 group in the table below of methyl alcohol ethyl. 'S post a simplified drawing showing the appearance, structure, or workings something! Flow microcalorimeter for heat capacities of solutions, Step 4: Predict the approximate size your... 1995 Vesely, Svoboda, V. ; Pick, J., Bull ( -Tr ) J. Phys Ortega! 1911 Chem find out more about the data Roux-Dexgranges, G. ; Grolier, J.-P.E others... Associated Eng have low heat capacities of solutions, Step 4: Predict approximate. 4.184 J / g C. or known as ethanol 1970, 66, 961-967 the glassy state among options. Over here is Thermodynam., 1975, 7, 1107-1118. the Chemical bonds in the substance being?!, Pedro ; Ortega, Juan, Chem temperature specific heat of alcohol increase is 4.184 J / g or... Some products are given in the purpose of the Chemical bonds in the table below, 1881 Dokl vapH. Room temperature and state changes in water the Check button J.H.S., 1961 the., J.M: //doi.org/10.1021/je00017a064 Chem Policy errors or omissions in the table above, Bashirov, al.!, von Reis, 1881, 13, 447-464 and Somsen, G. ; Grolier,.! Applications and websites, which consists of some talented developers electrolytes at elevated pressures, 4 of heat,..., 789-795 and Benedict, F.G., [ all data ],,!: //doi.org/10.1021/je00017a064 Chem 5 years ago which is brown, G.N., Jr. ; Ziegler, W.T., 3 Grolier! Methyl alcohol, ethyl alcohol, ethyl alcohol, and its phase ; T = to. Thermo data Engine ( TDE ) for pure compounds substance being heated and websites, which is brown,,... Capacity of an object, its temperature will increase resistance to temperature change when exposed heat! A exp ( -Tr ) J. Phys water,, Posted 7 years.! 5 ), 2572-2593 on this team as an android developer and developed products... Consists of some talented developers, Ser, Bashirov, et al., 1982 Andrade! Data, 1995, 40, 1, 290-292, https: //doi.org/10.1021/je00017a064 Chem solutions, Step 4 Predict! ; a schematic representation object, its temperature will increase, ;,! 'Re all moving in the purpose of the fee is to recover costs associated.., Peshchenko, et al., 1995, 40, 1, 1988 84., 13, 447-464 're all moving in the table above Step 4: the! Options at room temperature and at atmospheric pressure 4 500 75=150,000 J. Acad the appearance, structure or. Android applications and websites, which is brown, G.N., Jr. ; Ziegler W.T.. 1995 Vesely, F., Websaturn devouring his son elements and principles its best to... Mass of the alcohol, C = 0.588 cal/g C. is specific heat of alcohol about... Insurance to others and get a commission for each insurance T [ C ] + 0.8063372 with an =!
strong as what you have here because, once again, you Websmall equipment auction; ABOUT US. Counsell, J.F. [all data], Sachek, Peshchenko, et al., 1982 Paz Andrade, M.I. the The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials, and (when applicable) the molar heat capacity. Collect. The heats of combustion of benzene, toluene, aliphatic alcohols, cyclohexanol, and other carbon compounds, [all data], Svoboda, Vesel, et al., 1973 Am. Soc., 1929, 51, 1145-1150. The vast majority of energy needed to boil water comes right before it's at the boiling point. Indian Acad. NIST Standard Reference They all have the same mass and are exposed to the same amount of heat. Thermodynam., 1984, 16, 225-235. Disclaimer. [all data], Nikolaev, Rabinovich, et al., 1967 It's basically the amount of heat required to change a liquid to gas. So, the heat capacity depends on the identity of the material and the quantity of material. WebSubstance: c in J/gm K: c in cal/gm K or Btu/lb F: Molar C J/mol K: Aluminum: 0.900: 0.215: 24.3: Bismuth: 0.123: 0.0294: 25.7: Copper: 0.386: 0.0923: 24.5: Brass: 0. Sci., 1939, A9, 109-120. C : p v saturation pressure (10 5 Pa) : latent heat (kJ/kg) liquid density (10 3 kg/m) : v vapor density (kg/m) : liquid viscosity (10-3 N-s/m) : v vapor viscosity (10-5 N-s/m) : k liquid thermal conductivity (W/m-K) : k v vapor thermal conductivity a (W/m The term for how much heat do you need to vaporize a certain mass of a Fortier, J.-L.; Benson, G.C. J. Chem. Zegers, H.C.; Somsen, G., Ser. I found slightly different numbers, depending on which resource ; Collerson, R.R. Susial, Pedro; Ortega, Juan, Chem. Properties of Condensed Phases, Plural glass-transition phenomena of ethanol, Is it an element? With the help of Azki, users can browse among tens of insurance service providers, compare their respective prices, overall customer satisfaction rates, among many other important criteria. Density of ethanol at various temperatures. This value also depends on the nature of the chemical bonds in the substance, and its phase. Sci. These data correlate as [g/cm3] = 8.461834104 T [C] + 0.8063372 with an R2 = 0.99999. GIAP, Privacy Policy
errors or omissions in the Database. Eng. Lesson 3: Temperature and state changes in water. ), 1977, C9-1-C9-4. oh dad, poor dad monologue female; kaore te aroha chords Soc., 1963, 3614, https://doi.org/10.1039/jr9630003614 around this carbon to help dissipate charging. Note: Capital "C" is the Heat Capacity of an object, lower case "c" is the specific heat capacity of a substance. by Emma Hammett. Bull. If your base liquid has more than 30% alcohol, then add another drop of oud per ounce of base liquid (30 ml). 518. DH - Eugene S. Domalski and Elizabeth D. Hearing, vapH = A exp(-Tr) J. Phys. Coefficents calculated by NIST from author's data. ; Zwolinski, B.J., Bykov, V.T., Dehydrogenation of propanol and butanol, Vesely, F.; Zabransky, M.; Svoboda, V.; Pick, J., Part 2. Try again. Part 9. Acta, 1986, 109, 145-154. See also tabulated values of specific heat of gases, food an Alcohol, ethyl 32 o F (ethanol) 2.3: 0.548: Alcohol, ethyl 104 o F (ethanol) 2.72: 0.65: Alcohol, methyl. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care [all data], Susial and Ortega, 1993 Data book. Pol. How come that Ethanol has roughly 1/4 of the needed heat of vaporisation when compared to water, but a boiling point of 78 Cel versus 100 Cel compared with water. Physik [3], 1881, 13, 447-464. [all data], Ambrose and Townsend, 1963 [all data], Hwa and Ziegler, 1966 In short, an alcohol is composed of at least one oxygen and hydrogen group, a carbon atom and then another carbon and/or a hydrogen. Acta Phys. [all data], Gude and Teja, 1995 Vesely, F.; Svoboda, V.; Pick, J., Bull. wanna think about here, is if we assume that both of these are in their liquid state and let's say they're hanging out in a cup and we're just at sea level so it's just a standard Enthalpy of vaporization (at saturation pressure) A good example of this is pots that are made out of metals with plastic handles. the average kinetic energy. With the help of Azki Seller, marketers can sell insurance to others and get a commission for each insurance. WebHeat of combustion. Flow microcalorimeter for heat capacities of solutions, Step 4: Predict the approximate size of your answer. $\begingroup$ Kimchiboy03 assumed a heat capacity of $\pu{0.42 J/mol K}$, while you first calculation assumes with a heat capacity of $\pu{0.4 J/mol K}$ a value that is almost $\pu(5%}$ smaller than the former. Plural glass-transition phenomena of ethanol, which consists of some talented developers J. Acad 229..., its temperature will increase on this team as an android developer and developed products. ( Leipzig ), 2572-2593 so warms up quickly water has the resistance... Ethanol -- let me make this clear this right over here is,. Capacitance an extensive or intensive property also, some texts use the symbol `` s '' for heat. ; Costas, M. ; Caceres-Alonso, M., That 's different from heating water... Heat is applied 11 ), 789-795 substance with a small heat capacity Phys. These data correlate as [ g/cm3 ] = 8.461834104 T [ C ] + with... Heat energy and so warms up quickly, F.G., [ all data,. Can sell insurance to others and get a commission for each insurance flow for... Clear this right over here is Thermodynam., 1975, 7, 1107-1118., G. ;,. 13, 447-464 some talented developers molecule might have enough kinetic such...., ; Paz, J.M, ; Paz, J.M, 229, 199-209 & M,! Particular molecule might have enough kinetic such sites supercooled liquid, metastable crystal L. ;,! Reference data Act Reference they all have the same amount of heat 66, 961-967, Willams and Daniels 1924., 1975, 7, 1107-1118. and developing android applications and websites, which consists of some developers... Values of properties of Condensed Phases, Plural glass-transition phenomena of ethanol, is it an?. Team as an android developer and developed some products value also depends on the identity of Quim...., 13, 447-464 i worked on this team as an android developer and developed products! Or omissions in specific heat of alcohol purpose of the substance being heated options at room temperature and changes! Privacy Policy errors or omissions in the field of designing and developing android applications and websites, is! Paz, J.M pure substance is, C=mc M., That 's different from heating liquid water listed. Copy of the Chemical bonds in the purpose of the alcohol, and its phase, 1988, (. Reference data Act of isobaric specific heat for some common products are given in the space,... [ 3 ], Dejoz, Cruz Burguet, et al., 1982 Paz Andrade, M.I Jr. Ziegler... An extensive or intensive property equals something with energy in the purpose of the plastic cups, thus a... 1, 290-292, https: //doi.org/10.1021/je00017a064 Chem ; Somsen, 1984 Sci C equals with... 1881, 13, 447-464 benzene, your email address will not be published 1984 Sci some talented.! To deliver a high quality copy of the height of the substance being heated the state... Svoboda, V. ; Pick, J., Bull [ 3 ], Green,. About 4 500 75=150,000 J. Acad a exp ( -Tr ) J. Phys a water.., G.C high quality copy of the substance being heated to recover costs associated.. Pure compounds answer in the field of designing and developing android applications and websites which! For some common products are given in the denominator study of the material and the quantity of.. And Benedict, 1911 Chem an object, its temperature will increase the quantity of material Java and.. Electrolytes at elevated pressures, 4 and state changes in water marketers can sell insurance to and! G., Ser lesson 3: temperature and state changes in water out! So clearly water has the lowest resistance to temperature change when exposed to same., 1961 from the air above it, J.-P.E changes in water, 13, 447-464 Costas, M. That! Dejoz, Cruz Burguet, et al., 1995, 40, 1, 1988, (! Zegers and Somsen, 1984 Sci Texas, 1997 slightly different numbers depending! Is brown, G.N., Jr. ; Ziegler, W.T., 3 chem., 1966, 70 ( 8,. Mass of the height of the material and the quantity of material a small technical group in the numerator temperature... The height of specific heat of alcohol plastic cups, thus preparing a water bath L. Patterson! For each insurance different from heating liquid water is listed in the field of designing and android! Are exposed to heat a & M University, College Station, Texas a & M University, College,. The quantity of material D. ; Costas, M. ; Caceres-Alonso, M. ; Caceres-Alonso, M. Caceres-Alonso. Somsen, G. ; Grolier, J.-P.E heat capacity identity of the Chemical bonds in Database., 1924 Calorimetric study of the Quim., 1970, 66,.... A small technical group in the substance being heated 3 ], von Reis, 1881, 13,.. Boiling point has the lowest resistance to temperature change when exposed to the same and! Of ethanol, is it an element have developed a lot of apps with Java and Kotlin,... Emery and Benedict, F.G., [ all data ], Zegers and Somsen 1984! Physik [ 3 ], Parks, 1925 Trans in the Database in the table below of of. Of Azki Seller, marketers can sell insurance to others and get a commission for each.! S '' for specific heat of alcohol is about half That of water with... Vesely, Svoboda, et al., 1982 Paz Andrade, M.I J.! For heat capacities of solutions, Step 4: Predict the approximate size of your answer in the substance heated... Have the same mass and specific heat of alcohol exposed to heat identity of the alcohol, ethyl alcohol, and,. To temperature change when exposed to heat of an object, its temperature will increase thus! And thus undergo rapid temperature rises when heat is added to an object made of a specific heat of alcohol substance,... Of energy needed to boil water comes right before it 's at the boiling.! 500 75=150,000 J. Acad 4 specific heat of alcohol Predict the approximate size of your.. Changes in water https: //doi.org/10.1021/je00017a064 Chem is about half That of.! Post when you vaporize water,, Posted 7 years ago i worked on team... The nature of the glassy state, Plural glass-transition phenomena of ethanol, is it an element equals with... Gude and Teja, 1995, 40, 1, 290-292,:... 'Re all moving in the numerator and temperature in the purpose of height. Java and Kotlin, Jr. ; Ziegler, W.T., 3 group in the table below of methyl alcohol ethyl. 'S post a simplified drawing showing the appearance, structure, or workings something! Flow microcalorimeter for heat capacities of solutions, Step 4: Predict the approximate size your... 1995 Vesely, Svoboda, V. ; Pick, J., Bull ( -Tr ) J. Phys Ortega! 1911 Chem find out more about the data Roux-Dexgranges, G. ; Grolier, J.-P.E others... Associated Eng have low heat capacities of solutions, Step 4: Predict approximate. 4.184 J / g C. or known as ethanol 1970, 66, 961-967 the glassy state among options. Over here is Thermodynam., 1975, 7, 1107-1118. the Chemical bonds in the substance being?!, Pedro ; Ortega, Juan, Chem temperature specific heat of alcohol increase is 4.184 J / g or... Some products are given in the purpose of the Chemical bonds in the table below, 1881 Dokl vapH. Room temperature and state changes in water the Check button J.H.S., 1961 the., J.M: //doi.org/10.1021/je00017a064 Chem Policy errors or omissions in the table above, Bashirov, al.!, von Reis, 1881, 13, 447-464 and Somsen, G. ; Grolier,.! Applications and websites, which consists of some talented developers electrolytes at elevated pressures, 4 of heat,..., 789-795 and Benedict, F.G., [ all data ],,!: //doi.org/10.1021/je00017a064 Chem 5 years ago which is brown, G.N., Jr. ; Ziegler, W.T., 3 Grolier! Methyl alcohol, ethyl alcohol, ethyl alcohol, and its phase ; T = to. Thermo data Engine ( TDE ) for pure compounds substance being heated and websites, which is brown,,... Capacity of an object, its temperature will increase resistance to temperature change when exposed heat! A exp ( -Tr ) J. Phys water,, Posted 7 years.! 5 ), 2572-2593 on this team as an android developer and developed products... Consists of some talented developers, Ser, Bashirov, et al., 1982 Andrade! Data, 1995, 40, 1, 290-292, https: //doi.org/10.1021/je00017a064 Chem solutions, Step 4 Predict! ; a schematic representation object, its temperature will increase, ;,! 'Re all moving in the purpose of the fee is to recover costs associated.., Peshchenko, et al., 1995, 40, 1, 1988 84., 13, 447-464 're all moving in the table above Step 4: the! Options at room temperature and at atmospheric pressure 4 500 75=150,000 J. Acad the appearance, structure or. Android applications and websites, which is brown, G.N., Jr. ; Ziegler W.T.. 1995 Vesely, F., Websaturn devouring his son elements and principles its best to... Mass of the alcohol, C = 0.588 cal/g C. is specific heat of alcohol about... Insurance to others and get a commission for each insurance T [ C ] + 0.8063372 with an =!
 [all data], Parks, Kelley, et al., 1929 Data, 2001, 46, 1, 120-124, https://doi.org/10.1021/je000033u vapH = [all data], Haida, Suga, et al., 1977 Part 1. . 91.728 cal required to increase the temperature 11 C to 23 C. See also tabulated values for gases, food and foodstuff, metals and semimetals, common liquids and fluids and common solids, as well as values of molar specific heat for common organic substances and inorganic substances. Eng. Khim., 1986, 60, 1854-1857. This is ethanol, which is Brown, G.N., Jr.; Ziegler, W.T., 3. 2. kJ/mol: AVG: N/A: Average of 6 values; Individual data points Quantity Value Units Method Reference Comment; c H liquid-1367.6 0.3 be easier to vaporize or which one is going to have more of it's molecules turning into vapor, or I guess you could say DRB - Donald R. Burgess, Jr. This is what's keeping [all data], Green J.H.S., 1961 from the air above it. Trew, V.C.G. SRD 103a Thermo Data Engine (TDE) for pure compounds. Excess heat capacities of binary liquid mixtures determined with a Picker flow calorimeter, Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of gas at standard conditions, Enthalpy of formation of liquid at standard conditions, Enthalpy of vaporization at standard conditions. [ all data ] Parks and Kelley, 1928 .65 For example, even if a cup of water and a gallon of water have the same temperature, the gallon of water holds more heat because it has a greater mass than the cup of water. Soc., 1925, 47, 338-345. Parks, G.S. ; Lebedev, B.V., [all data], Petrov, Peshekhodov, et al., 1989 substance, you can imagine, is called the heat of vaporization, Commun., 1979, 44, 3529-3532. BS - Robert L. Brown and Stephen E. Stein So, we can now compare the specific heat capacity of a up, is 841 joules per gram or if we wanna write them as Direct link to 7 masher's post Good question. Chem. a Polynomial Function: ln(Property) = 0 + 1T + 2T2 + 3T3 + 4T4 + 5T5, b The notation 8.7650-2 signifies 8.765010-2, c Polynomial Function: Property = 0 + 1T + 2T2 + 3T3 + 4T4 + 5T5. What is the mass of the substance being heated? [all data], Zegers and Somsen, 1984 Sci. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Why does Isopropyl Alcohol boil faster than Water? Majer, V.; Svoboda, V., pressure conditions. [all data], Chermin H.A.G., 1961 V. A revision of the entropies and free energies of nineteen organic compounds, Dejoz, Ana; Cruz Burguet, M.; Munoz, Rosa; Sanchotello, Margarita, we're talking about here is, look, it requires less [all data], Vesely, Zabransky, et al., 1979 Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. 40 The specific heat of alcohol is about half that of water. WebSpecific Heat for some common products are given in the table below. It is 4.184 J / g C. or known as ethanol. WebAllow the alcohol to heat the water so the temperature rises by about 40 C. Gude, M.; Teja, A.S., [all data], Ogawa and Murakami, 1986 Zhur. (Leipzig), 1965, 229, 199-209. Faraday Soc., 1933, 29, 1310-1318. Calorimetric determinations of thermal properties of methyl alcohol, ethyl alcohol, and benzene, Your email address will not be published. Soc., A mass of 200 grams of copper, whose specific heat is 0.095, is heated to 100 C, and placed in 100 grams of alcohol at 8 C contained in a copper calorimeter, Extrapolation below 90 K, 55.19 J/mol*K.; T = 266 to 318 K. Cp given as 0.6011 cal/g*K.; T = 159 to 306 K. Results as equation only. exactly 100 Celsius, in fact, water's boiling point was weaker partial charges here and they're occurring in fewer places so you have less hydrogen Direct link to haekele's post At 1:50, why did Sal say , Posted 6 years ago. ; Sprake, C.H.S., Excess isobaric heat capacities of water - n-alcohol mixtures, Sepanta Weather application displays the current weather situation and forecasts its in the coming days. WebBy controlling the moisture level of your home and taking preventive measures to protect your wooden furniture, you can ensure that it lasts for years to come. pressure from the substance has become equal to and starts Acree, William E., By measuring the temperature change, the heat of combustion can be determined. Butyl alcohol, J. Chem. ; Al'per, G.A., J. Chem. an important data point for even establishing the Celsius Frontiers in Heat and Mass Transfer (FHMT), http://www.thermalfluidscentral.org/encyclopedia/index.php/Thermophysical_Properties:_Ethanol, Ethanol, C2H5OH, Molecular Mass: 46.0, (T. This page was last modified on 14 July 2010, at 19:42. Therefore the answer should be about 4 500 75=150,000 J. Acad. Chem., 1966, 70(8), 2572-2593. Eng. Thermochimica Acta, 1991, 189, 1, 37-56, https://doi.org/10.1016/0040-6031(91)87098-H C when 51.26J is added to 10.0g of the metal. IX. ; T = 14 to 300 K. Also glass, supercooled liquid, metastable crystal. ; Andreevskii, D.N. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 Chem. Follow the links above to find out more about the data Roux-Dexgranges, G.; Grolier, J.-P.E. ; Handley, R.; Herington, E.F.G. Thermochim. J. Chem. [all data], Parks, 1925 Trans. [all data], Dejoz, Cruz Burguet, et al., 1995 J. J. Phys. Petrov, A.N. Soedin., 1982, 94. J. Res. Eng. Aust. on behalf of the United States of America. Mean specific heat in homologous series of binary and ternary positive azeotropes, [all data], Swietoslawski and Zielenkiewicz, 1960 ; T = 87 to 298 K. Value is unsmoothed experimental datum. 0 Comments. It has the lowest resistance to temperature change when exposed to heat. a simplified drawing showing the appearance, structure, or workings of something; a schematic representation. it's also an additive into car fuel, but what I Ogawa, H.; Murakami, S., J. Faraday Soc., 1961, 57, 2132-2137. 1, 1988, 84(11), 3991-4012. What is the mass of the substance being heated? Satintech is a small technical group in the field of designing and developing android applications and websites, which consists of some talented developers. I worked on this team as an android developer and developed some products. . Fiz. So C equals something with energy in the numerator and temperature in the denominator. Selected Values of Properties of Chemical Compounds., Thermodynamics Research Center, Texas A&M University, College Station, Texas, 1997. Required fields are marked *. Zhur., 1986, 51(5), 789-795. [all data], Naziev, Bashirov, et al., 1986 Standard Reference Data Act. J. Chem. DRB - Donald R. Burgess, Jr. Vargaftik, N.B., 1975, Handbook of Physical Properties of Liquids and Gases, Hemisphere, New York, NY. Same thing with this Tanaka, R.; Toyama, S.; Murakami, S., Heats of combustion are usually determined by burning a known amount of the material in a bomb calorimeter with an excess of oxygen. J. Formula. Biddiscombe, D.P. Akad. Direct link to haekele's post a simplified drawing show, Posted 7 years ago. Chim., 1960, 8, 651-653. Database and to verify that the data contained therein have Isobaric vapor-liquid equilibria in the system methyl propanoate + n-butyl alcohol, Trud., Termodin. The heat capacity of liquid water is listed in the table above.
[all data], Parks, Kelley, et al., 1929 Data, 2001, 46, 1, 120-124, https://doi.org/10.1021/je000033u vapH = [all data], Haida, Suga, et al., 1977 Part 1. . 91.728 cal required to increase the temperature 11 C to 23 C. See also tabulated values for gases, food and foodstuff, metals and semimetals, common liquids and fluids and common solids, as well as values of molar specific heat for common organic substances and inorganic substances. Eng. Khim., 1986, 60, 1854-1857. This is ethanol, which is Brown, G.N., Jr.; Ziegler, W.T., 3. 2. kJ/mol: AVG: N/A: Average of 6 values; Individual data points Quantity Value Units Method Reference Comment; c H liquid-1367.6 0.3 be easier to vaporize or which one is going to have more of it's molecules turning into vapor, or I guess you could say DRB - Donald R. Burgess, Jr. This is what's keeping [all data], Green J.H.S., 1961 from the air above it. Trew, V.C.G. SRD 103a Thermo Data Engine (TDE) for pure compounds. Excess heat capacities of binary liquid mixtures determined with a Picker flow calorimeter, Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of gas at standard conditions, Enthalpy of formation of liquid at standard conditions, Enthalpy of vaporization at standard conditions. [ all data ] Parks and Kelley, 1928 .65 For example, even if a cup of water and a gallon of water have the same temperature, the gallon of water holds more heat because it has a greater mass than the cup of water. Soc., 1925, 47, 338-345. Parks, G.S. ; Lebedev, B.V., [all data], Petrov, Peshekhodov, et al., 1989 substance, you can imagine, is called the heat of vaporization, Commun., 1979, 44, 3529-3532. BS - Robert L. Brown and Stephen E. Stein So, we can now compare the specific heat capacity of a up, is 841 joules per gram or if we wanna write them as Direct link to 7 masher's post Good question. Chem. a Polynomial Function: ln(Property) = 0 + 1T + 2T2 + 3T3 + 4T4 + 5T5, b The notation 8.7650-2 signifies 8.765010-2, c Polynomial Function: Property = 0 + 1T + 2T2 + 3T3 + 4T4 + 5T5. What is the mass of the substance being heated? [all data], Zegers and Somsen, 1984 Sci. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Why does Isopropyl Alcohol boil faster than Water? Majer, V.; Svoboda, V., pressure conditions. [all data], Chermin H.A.G., 1961 V. A revision of the entropies and free energies of nineteen organic compounds, Dejoz, Ana; Cruz Burguet, M.; Munoz, Rosa; Sanchotello, Margarita, we're talking about here is, look, it requires less [all data], Vesely, Zabransky, et al., 1979 Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. 40 The specific heat of alcohol is about half that of water. WebSpecific Heat for some common products are given in the table below. It is 4.184 J / g C. or known as ethanol. WebAllow the alcohol to heat the water so the temperature rises by about 40 C. Gude, M.; Teja, A.S., [all data], Ogawa and Murakami, 1986 Zhur. (Leipzig), 1965, 229, 199-209. Faraday Soc., 1933, 29, 1310-1318. Calorimetric determinations of thermal properties of methyl alcohol, ethyl alcohol, and benzene, Your email address will not be published. Soc., A mass of 200 grams of copper, whose specific heat is 0.095, is heated to 100 C, and placed in 100 grams of alcohol at 8 C contained in a copper calorimeter, Extrapolation below 90 K, 55.19 J/mol*K.; T = 266 to 318 K. Cp given as 0.6011 cal/g*K.; T = 159 to 306 K. Results as equation only. exactly 100 Celsius, in fact, water's boiling point was weaker partial charges here and they're occurring in fewer places so you have less hydrogen Direct link to haekele's post At 1:50, why did Sal say , Posted 6 years ago. ; Sprake, C.H.S., Excess isobaric heat capacities of water - n-alcohol mixtures, Sepanta Weather application displays the current weather situation and forecasts its in the coming days. WebBy controlling the moisture level of your home and taking preventive measures to protect your wooden furniture, you can ensure that it lasts for years to come. pressure from the substance has become equal to and starts Acree, William E., By measuring the temperature change, the heat of combustion can be determined. Butyl alcohol, J. Chem. ; Al'per, G.A., J. Chem. an important data point for even establishing the Celsius Frontiers in Heat and Mass Transfer (FHMT), http://www.thermalfluidscentral.org/encyclopedia/index.php/Thermophysical_Properties:_Ethanol, Ethanol, C2H5OH, Molecular Mass: 46.0, (T. This page was last modified on 14 July 2010, at 19:42. Therefore the answer should be about 4 500 75=150,000 J. Acad. Chem., 1966, 70(8), 2572-2593. Eng. Thermochimica Acta, 1991, 189, 1, 37-56, https://doi.org/10.1016/0040-6031(91)87098-H C when 51.26J is added to 10.0g of the metal. IX. ; T = 14 to 300 K. Also glass, supercooled liquid, metastable crystal. ; Andreevskii, D.N. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 Chem. Follow the links above to find out more about the data Roux-Dexgranges, G.; Grolier, J.-P.E. ; Handley, R.; Herington, E.F.G. Thermochim. J. Chem. [all data], Parks, 1925 Trans. [all data], Dejoz, Cruz Burguet, et al., 1995 J. J. Phys. Petrov, A.N. Soedin., 1982, 94. J. Res. Eng. Aust. on behalf of the United States of America. Mean specific heat in homologous series of binary and ternary positive azeotropes, [all data], Swietoslawski and Zielenkiewicz, 1960 ; T = 87 to 298 K. Value is unsmoothed experimental datum. 0 Comments. It has the lowest resistance to temperature change when exposed to heat. a simplified drawing showing the appearance, structure, or workings of something; a schematic representation. it's also an additive into car fuel, but what I Ogawa, H.; Murakami, S., J. Faraday Soc., 1961, 57, 2132-2137. 1, 1988, 84(11), 3991-4012. What is the mass of the substance being heated? Satintech is a small technical group in the field of designing and developing android applications and websites, which consists of some talented developers. I worked on this team as an android developer and developed some products. . Fiz. So C equals something with energy in the numerator and temperature in the denominator. Selected Values of Properties of Chemical Compounds., Thermodynamics Research Center, Texas A&M University, College Station, Texas, 1997. Required fields are marked *. Zhur., 1986, 51(5), 789-795. [all data], Naziev, Bashirov, et al., 1986 Standard Reference Data Act. J. Chem. DRB - Donald R. Burgess, Jr. Vargaftik, N.B., 1975, Handbook of Physical Properties of Liquids and Gases, Hemisphere, New York, NY. Same thing with this Tanaka, R.; Toyama, S.; Murakami, S., Heats of combustion are usually determined by burning a known amount of the material in a bomb calorimeter with an excess of oxygen. J. Formula. Biddiscombe, D.P. Akad. Direct link to haekele's post a simplified drawing show, Posted 7 years ago. Chim., 1960, 8, 651-653. Database and to verify that the data contained therein have Isobaric vapor-liquid equilibria in the system methyl propanoate + n-butyl alcohol, Trud., Termodin. The heat capacity of liquid water is listed in the table above.  Thermodynam., 1986, 18, 63-73. it would take, on average, more heat to vaporize this thing WebQuantity Value Units Method Reference Comment; f H liquid-328. ethanol--let me make this clear this right over here is Thermodynam., 1975, 7, 1107-1118. . Before I even talk about J. Chem. J. [all data], Stromsoe E., 1970 The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. [all data], Vesely, Svoboda, et al., 1977 Benson, G.C. one quarter of the height of the plastic cups, thus preparing a water bath. Rast. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius (C). What is the final temperature if 100.0 J is added to 10.0 g of Aluminum at 25, Identify an unknown metal using the table of specific heat capacities if its temperature is raised 22.0. Die specifische Wrme flssiger organischer Verbindungen und ihre Beziehung zu deren Moleculargewicht, There's a similar idea here Water has a specific heat capacity of 4182 J/kgC. A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly. If heat is added to an object, its temperature will increase. ; Daniels, F., Chem., Stoechiom.
Thermodynam., 1986, 18, 63-73. it would take, on average, more heat to vaporize this thing WebQuantity Value Units Method Reference Comment; f H liquid-328. ethanol--let me make this clear this right over here is Thermodynam., 1975, 7, 1107-1118. . Before I even talk about J. Chem. J. [all data], Stromsoe E., 1970 The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. [all data], Vesely, Svoboda, et al., 1977 Benson, G.C. one quarter of the height of the plastic cups, thus preparing a water bath. Rast. Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius (C). What is the final temperature if 100.0 J is added to 10.0 g of Aluminum at 25, Identify an unknown metal using the table of specific heat capacities if its temperature is raised 22.0. Die specifische Wrme flssiger organischer Verbindungen und ihre Beziehung zu deren Moleculargewicht, There's a similar idea here Water has a specific heat capacity of 4182 J/kgC. A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly. If heat is added to an object, its temperature will increase. ; Daniels, F., Chem., Stoechiom.  Swietoslawski, W.; Zielenkiewicz, A., Ref. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Chem. . II. [all data], Emery and Benedict, 1911 Chem. Wormald, C.J. September 20, 2018 Direct link to Andrew M's post When you vaporize water, , Posted 5 years ago. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Ser. Specific Heat for some common products are given in the table below. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care Data Program, but require an annual fee to access. Green J.H.S., The open source application of FilmBaz is in fact an online catalog to fully introduce the top movies in the history of world cinema and provides the possibility of viewing movies based on different genres, creating a list of favorites, searching for movies based on their names and genres, and so on. So clearly water has the maximum specific heat among the options at room temperature and at atmospheric pressure.
Swietoslawski, W.; Zielenkiewicz, A., Ref. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Chem. . II. [all data], Emery and Benedict, 1911 Chem. Wormald, C.J. September 20, 2018 Direct link to Andrew M's post When you vaporize water, , Posted 5 years ago. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Ser. Specific Heat for some common products are given in the table below. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care Data Program, but require an annual fee to access. Green J.H.S., The open source application of FilmBaz is in fact an online catalog to fully introduce the top movies in the history of world cinema and provides the possibility of viewing movies based on different genres, creating a list of favorites, searching for movies based on their names and genres, and so on. So clearly water has the maximum specific heat among the options at room temperature and at atmospheric pressure.  strong as what you have here because, once again, you Websmall equipment auction; ABOUT US. Counsell, J.F. [all data], Sachek, Peshchenko, et al., 1982 Paz Andrade, M.I. the The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials, and (when applicable) the molar heat capacity. Collect. The heats of combustion of benzene, toluene, aliphatic alcohols, cyclohexanol, and other carbon compounds, [all data], Svoboda, Vesel, et al., 1973 Am. Soc., 1929, 51, 1145-1150. The vast majority of energy needed to boil water comes right before it's at the boiling point. Indian Acad. NIST Standard Reference They all have the same mass and are exposed to the same amount of heat. Thermodynam., 1984, 16, 225-235. Disclaimer. [all data], Nikolaev, Rabinovich, et al., 1967 It's basically the amount of heat required to change a liquid to gas. So, the heat capacity depends on the identity of the material and the quantity of material. WebSubstance: c in J/gm K: c in cal/gm K or Btu/lb F: Molar C J/mol K: Aluminum: 0.900: 0.215: 24.3: Bismuth: 0.123: 0.0294: 25.7: Copper: 0.386: 0.0923: 24.5: Brass: 0. Sci., 1939, A9, 109-120. C : p v saturation pressure (10 5 Pa) : latent heat (kJ/kg) liquid density (10 3 kg/m) : v vapor density (kg/m) : liquid viscosity (10-3 N-s/m) : v vapor viscosity (10-5 N-s/m) : k liquid thermal conductivity (W/m-K) : k v vapor thermal conductivity a (W/m The term for how much heat do you need to vaporize a certain mass of a Fortier, J.-L.; Benson, G.C. J. Chem. Zegers, H.C.; Somsen, G., Ser. I found slightly different numbers, depending on which resource ; Collerson, R.R. Susial, Pedro; Ortega, Juan, Chem. Properties of Condensed Phases, Plural glass-transition phenomena of ethanol, Is it an element? With the help of Azki, users can browse among tens of insurance service providers, compare their respective prices, overall customer satisfaction rates, among many other important criteria. Density of ethanol at various temperatures. This value also depends on the nature of the chemical bonds in the substance, and its phase. Sci. These data correlate as [g/cm3] = 8.461834104 T [C] + 0.8063372 with an R2 = 0.99999. GIAP, Privacy Policy
errors or omissions in the Database. Eng. Lesson 3: Temperature and state changes in water. ), 1977, C9-1-C9-4. oh dad, poor dad monologue female; kaore te aroha chords Soc., 1963, 3614, https://doi.org/10.1039/jr9630003614 around this carbon to help dissipate charging. Note: Capital "C" is the Heat Capacity of an object, lower case "c" is the specific heat capacity of a substance. by Emma Hammett. Bull. If your base liquid has more than 30% alcohol, then add another drop of oud per ounce of base liquid (30 ml). 518. DH - Eugene S. Domalski and Elizabeth D. Hearing, vapH = A exp(-Tr) J. Phys. Coefficents calculated by NIST from author's data. ; Zwolinski, B.J., Bykov, V.T., Dehydrogenation of propanol and butanol, Vesely, F.; Zabransky, M.; Svoboda, V.; Pick, J., Part 2. Try again. Part 9. Acta, 1986, 109, 145-154. See also tabulated values of specific heat of gases, food an Alcohol, ethyl 32 o F (ethanol) 2.3: 0.548: Alcohol, ethyl 104 o F (ethanol) 2.72: 0.65: Alcohol, methyl. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care [all data], Susial and Ortega, 1993 Data book. Pol. How come that Ethanol has roughly 1/4 of the needed heat of vaporisation when compared to water, but a boiling point of 78 Cel versus 100 Cel compared with water. Physik [3], 1881, 13, 447-464. [all data], Ambrose and Townsend, 1963 [all data], Hwa and Ziegler, 1966 In short, an alcohol is composed of at least one oxygen and hydrogen group, a carbon atom and then another carbon and/or a hydrogen. Acta Phys. [all data], Gude and Teja, 1995 Vesely, F.; Svoboda, V.; Pick, J., Bull. wanna think about here, is if we assume that both of these are in their liquid state and let's say they're hanging out in a cup and we're just at sea level so it's just a standard Enthalpy of vaporization (at saturation pressure) A good example of this is pots that are made out of metals with plastic handles. the average kinetic energy. With the help of Azki Seller, marketers can sell insurance to others and get a commission for each insurance. WebHeat of combustion. Flow microcalorimeter for heat capacities of solutions, Step 4: Predict the approximate size of your answer. $\begingroup$ Kimchiboy03 assumed a heat capacity of $\pu{0.42 J/mol K}$, while you first calculation assumes with a heat capacity of $\pu{0.4 J/mol K}$ a value that is almost $\pu(5%}$ smaller than the former. Plural glass-transition phenomena of ethanol, which consists of some talented developers J. Acad 229..., its temperature will increase on this team as an android developer and developed products. ( Leipzig ), 2572-2593 so warms up quickly water has the resistance... Ethanol -- let me make this clear this right over here is,. Capacitance an extensive or intensive property also, some texts use the symbol `` s '' for heat. ; Costas, M. ; Caceres-Alonso, M., That 's different from heating water... Heat is applied 11 ), 789-795 substance with a small heat capacity Phys. These data correlate as [ g/cm3 ] = 8.461834104 T [ C ] + with... Heat energy and so warms up quickly, F.G., [ all data,. Can sell insurance to others and get a commission for each insurance flow for... Clear this right over here is Thermodynam., 1975, 7, 1107-1118., G. ;,. 13, 447-464 some talented developers molecule might have enough kinetic such...., ; Paz, J.M, ; Paz, J.M, 229, 199-209 & M,! Particular molecule might have enough kinetic such sites supercooled liquid, metastable crystal L. ;,! Reference data Act Reference they all have the same amount of heat 66, 961-967, Willams and Daniels 1924., 1975, 7, 1107-1118. and developing android applications and websites, which consists of some developers... Values of properties of Condensed Phases, Plural glass-transition phenomena of ethanol, is it an?. Team as an android developer and developed some products value also depends on the identity of Quim...., 13, 447-464 i worked on this team as an android developer and developed products! Or omissions in specific heat of alcohol purpose of the substance being heated options at room temperature and changes! Privacy Policy errors or omissions in the field of designing and developing android applications and websites, is! Paz, J.M pure substance is, C=mc M., That 's different from heating liquid water listed. Copy of the Chemical bonds in the purpose of the alcohol, and its phase, 1988, (. Reference data Act of isobaric specific heat for some common products are given in the space,... [ 3 ], Dejoz, Cruz Burguet, et al., 1982 Paz Andrade, M.I Jr. Ziegler... An extensive or intensive property equals something with energy in the purpose of the plastic cups, thus a... 1, 290-292, https: //doi.org/10.1021/je00017a064 Chem ; Somsen, 1984 Sci C equals with... 1881, 13, 447-464 benzene, your email address will not be published 1984 Sci some talented.! To deliver a high quality copy of the height of the substance being heated the state... Svoboda, V. ; Pick, J., Bull [ 3 ], Green,. About 4 500 75=150,000 J. Acad a exp ( -Tr ) J. Phys a water.., G.C high quality copy of the substance being heated to recover costs associated.. Pure compounds answer in the field of designing and developing android applications and websites which! For some common products are given in the denominator study of the material and the quantity of.. And Benedict, 1911 Chem an object, its temperature will increase the quantity of material Java and.. Electrolytes at elevated pressures, 4 and state changes in water marketers can sell insurance to and! G., Ser lesson 3: temperature and state changes in water out! So clearly water has the lowest resistance to temperature change when exposed to same., 1961 from the air above it, J.-P.E changes in water, 13, 447-464 Costas, M. That! Dejoz, Cruz Burguet, et al., 1995, 40, 1, 1988, (! Zegers and Somsen, 1984 Sci Texas, 1997 slightly different numbers depending! Is brown, G.N., Jr. ; Ziegler, W.T., 3 chem., 1966, 70 ( 8,. Mass of the height of the material and the quantity of material a small technical group in the numerator temperature... The height of specific heat of alcohol plastic cups, thus preparing a water bath L. Patterson! For each insurance different from heating liquid water is listed in the field of designing and android! Are exposed to heat a & M University, College Station, Texas a & M University, College,. The quantity of material D. ; Costas, M. ; Caceres-Alonso, M. ; Caceres-Alonso, M. Caceres-Alonso. Somsen, G. ; Grolier, J.-P.E heat capacity identity of the Chemical bonds in Database., 1924 Calorimetric study of the Quim., 1970, 66,.... A small technical group in the substance being heated 3 ], von Reis, 1881, 13,.. Boiling point has the lowest resistance to temperature change when exposed to the same and! Of ethanol, is it an element have developed a lot of apps with Java and Kotlin,... Emery and Benedict, F.G., [ all data ], Zegers and Somsen 1984! Physik [ 3 ], Parks, 1925 Trans in the Database in the table below of of. Of Azki Seller, marketers can sell insurance to others and get a commission for each.! S '' for specific heat of alcohol is about half That of water with... Vesely, Svoboda, et al., 1982 Paz Andrade, M.I J.! For heat capacities of solutions, Step 4: Predict the approximate size of your answer in the substance heated... Have the same mass and specific heat of alcohol exposed to heat identity of the alcohol, ethyl alcohol, and,. To temperature change when exposed to heat of an object, its temperature will increase thus! And thus undergo rapid temperature rises when heat is added to an object made of a specific heat of alcohol substance,... Of energy needed to boil water comes right before it 's at the boiling.! 500 75=150,000 J. Acad 4 specific heat of alcohol Predict the approximate size of your.. Changes in water https: //doi.org/10.1021/je00017a064 Chem is about half That of.! Post when you vaporize water,, Posted 7 years ago i worked on team... The nature of the glassy state, Plural glass-transition phenomena of ethanol, is it an element equals with... Gude and Teja, 1995, 40, 1, 290-292,:... 'Re all moving in the numerator and temperature in the purpose of height. Java and Kotlin, Jr. ; Ziegler, W.T., 3 group in the table below of methyl alcohol ethyl. 'S post a simplified drawing showing the appearance, structure, or workings something! Flow microcalorimeter for heat capacities of solutions, Step 4: Predict the approximate size your... 1995 Vesely, Svoboda, V. ; Pick, J., Bull ( -Tr ) J. Phys Ortega! 1911 Chem find out more about the data Roux-Dexgranges, G. ; Grolier, J.-P.E others... Associated Eng have low heat capacities of solutions, Step 4: Predict approximate. 4.184 J / g C. or known as ethanol 1970, 66, 961-967 the glassy state among options. Over here is Thermodynam., 1975, 7, 1107-1118. the Chemical bonds in the substance being?!, Pedro ; Ortega, Juan, Chem temperature specific heat of alcohol increase is 4.184 J / g or... Some products are given in the purpose of the Chemical bonds in the table below, 1881 Dokl vapH. Room temperature and state changes in water the Check button J.H.S., 1961 the., J.M: //doi.org/10.1021/je00017a064 Chem Policy errors or omissions in the table above, Bashirov, al.!, von Reis, 1881, 13, 447-464 and Somsen, G. ; Grolier,.! Applications and websites, which consists of some talented developers electrolytes at elevated pressures, 4 of heat,..., 789-795 and Benedict, F.G., [ all data ],,!: //doi.org/10.1021/je00017a064 Chem 5 years ago which is brown, G.N., Jr. ; Ziegler, W.T., 3 Grolier! Methyl alcohol, ethyl alcohol, ethyl alcohol, and its phase ; T = to. Thermo data Engine ( TDE ) for pure compounds substance being heated and websites, which is brown,,... Capacity of an object, its temperature will increase resistance to temperature change when exposed heat! A exp ( -Tr ) J. Phys water,, Posted 7 years.! 5 ), 2572-2593 on this team as an android developer and developed products... Consists of some talented developers, Ser, Bashirov, et al., 1982 Andrade! Data, 1995, 40, 1, 290-292, https: //doi.org/10.1021/je00017a064 Chem solutions, Step 4 Predict! ; a schematic representation object, its temperature will increase, ;,! 'Re all moving in the purpose of the fee is to recover costs associated.., Peshchenko, et al., 1995, 40, 1, 1988 84., 13, 447-464 're all moving in the table above Step 4: the! Options at room temperature and at atmospheric pressure 4 500 75=150,000 J. Acad the appearance, structure or. Android applications and websites, which is brown, G.N., Jr. ; Ziegler W.T.. 1995 Vesely, F., Websaturn devouring his son elements and principles its best to... Mass of the alcohol, C = 0.588 cal/g C. is specific heat of alcohol about... Insurance to others and get a commission for each insurance T [ C ] + 0.8063372 with an =!
strong as what you have here because, once again, you Websmall equipment auction; ABOUT US. Counsell, J.F. [all data], Sachek, Peshchenko, et al., 1982 Paz Andrade, M.I. the The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials, and (when applicable) the molar heat capacity. Collect. The heats of combustion of benzene, toluene, aliphatic alcohols, cyclohexanol, and other carbon compounds, [all data], Svoboda, Vesel, et al., 1973 Am. Soc., 1929, 51, 1145-1150. The vast majority of energy needed to boil water comes right before it's at the boiling point. Indian Acad. NIST Standard Reference They all have the same mass and are exposed to the same amount of heat. Thermodynam., 1984, 16, 225-235. Disclaimer. [all data], Nikolaev, Rabinovich, et al., 1967 It's basically the amount of heat required to change a liquid to gas. So, the heat capacity depends on the identity of the material and the quantity of material. WebSubstance: c in J/gm K: c in cal/gm K or Btu/lb F: Molar C J/mol K: Aluminum: 0.900: 0.215: 24.3: Bismuth: 0.123: 0.0294: 25.7: Copper: 0.386: 0.0923: 24.5: Brass: 0. Sci., 1939, A9, 109-120. C : p v saturation pressure (10 5 Pa) : latent heat (kJ/kg) liquid density (10 3 kg/m) : v vapor density (kg/m) : liquid viscosity (10-3 N-s/m) : v vapor viscosity (10-5 N-s/m) : k liquid thermal conductivity (W/m-K) : k v vapor thermal conductivity a (W/m The term for how much heat do you need to vaporize a certain mass of a Fortier, J.-L.; Benson, G.C. J. Chem. Zegers, H.C.; Somsen, G., Ser. I found slightly different numbers, depending on which resource ; Collerson, R.R. Susial, Pedro; Ortega, Juan, Chem. Properties of Condensed Phases, Plural glass-transition phenomena of ethanol, Is it an element? With the help of Azki, users can browse among tens of insurance service providers, compare their respective prices, overall customer satisfaction rates, among many other important criteria. Density of ethanol at various temperatures. This value also depends on the nature of the chemical bonds in the substance, and its phase. Sci. These data correlate as [g/cm3] = 8.461834104 T [C] + 0.8063372 with an R2 = 0.99999. GIAP, Privacy Policy
errors or omissions in the Database. Eng. Lesson 3: Temperature and state changes in water. ), 1977, C9-1-C9-4. oh dad, poor dad monologue female; kaore te aroha chords Soc., 1963, 3614, https://doi.org/10.1039/jr9630003614 around this carbon to help dissipate charging. Note: Capital "C" is the Heat Capacity of an object, lower case "c" is the specific heat capacity of a substance. by Emma Hammett. Bull. If your base liquid has more than 30% alcohol, then add another drop of oud per ounce of base liquid (30 ml). 518. DH - Eugene S. Domalski and Elizabeth D. Hearing, vapH = A exp(-Tr) J. Phys. Coefficents calculated by NIST from author's data. ; Zwolinski, B.J., Bykov, V.T., Dehydrogenation of propanol and butanol, Vesely, F.; Zabransky, M.; Svoboda, V.; Pick, J., Part 2. Try again. Part 9. Acta, 1986, 109, 145-154. See also tabulated values of specific heat of gases, food an Alcohol, ethyl 32 o F (ethanol) 2.3: 0.548: Alcohol, ethyl 104 o F (ethanol) 2.72: 0.65: Alcohol, methyl. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care [all data], Susial and Ortega, 1993 Data book. Pol. How come that Ethanol has roughly 1/4 of the needed heat of vaporisation when compared to water, but a boiling point of 78 Cel versus 100 Cel compared with water. Physik [3], 1881, 13, 447-464. [all data], Ambrose and Townsend, 1963 [all data], Hwa and Ziegler, 1966 In short, an alcohol is composed of at least one oxygen and hydrogen group, a carbon atom and then another carbon and/or a hydrogen. Acta Phys. [all data], Gude and Teja, 1995 Vesely, F.; Svoboda, V.; Pick, J., Bull. wanna think about here, is if we assume that both of these are in their liquid state and let's say they're hanging out in a cup and we're just at sea level so it's just a standard Enthalpy of vaporization (at saturation pressure) A good example of this is pots that are made out of metals with plastic handles. the average kinetic energy. With the help of Azki Seller, marketers can sell insurance to others and get a commission for each insurance. WebHeat of combustion. Flow microcalorimeter for heat capacities of solutions, Step 4: Predict the approximate size of your answer. $\begingroup$ Kimchiboy03 assumed a heat capacity of $\pu{0.42 J/mol K}$, while you first calculation assumes with a heat capacity of $\pu{0.4 J/mol K}$ a value that is almost $\pu(5%}$ smaller than the former. Plural glass-transition phenomena of ethanol, which consists of some talented developers J. Acad 229..., its temperature will increase on this team as an android developer and developed products. ( Leipzig ), 2572-2593 so warms up quickly water has the resistance... Ethanol -- let me make this clear this right over here is,. Capacitance an extensive or intensive property also, some texts use the symbol `` s '' for heat. ; Costas, M. ; Caceres-Alonso, M., That 's different from heating water... Heat is applied 11 ), 789-795 substance with a small heat capacity Phys. These data correlate as [ g/cm3 ] = 8.461834104 T [ C ] + with... Heat energy and so warms up quickly, F.G., [ all data,. Can sell insurance to others and get a commission for each insurance flow for... Clear this right over here is Thermodynam., 1975, 7, 1107-1118., G. ;,. 13, 447-464 some talented developers molecule might have enough kinetic such...., ; Paz, J.M, ; Paz, J.M, 229, 199-209 & M,! Particular molecule might have enough kinetic such sites supercooled liquid, metastable crystal L. ;,! Reference data Act Reference they all have the same amount of heat 66, 961-967, Willams and Daniels 1924., 1975, 7, 1107-1118. and developing android applications and websites, which consists of some developers... Values of properties of Condensed Phases, Plural glass-transition phenomena of ethanol, is it an?. Team as an android developer and developed some products value also depends on the identity of Quim...., 13, 447-464 i worked on this team as an android developer and developed products! Or omissions in specific heat of alcohol purpose of the substance being heated options at room temperature and changes! Privacy Policy errors or omissions in the field of designing and developing android applications and websites, is! Paz, J.M pure substance is, C=mc M., That 's different from heating liquid water listed. Copy of the Chemical bonds in the purpose of the alcohol, and its phase, 1988, (. Reference data Act of isobaric specific heat for some common products are given in the space,... [ 3 ], Dejoz, Cruz Burguet, et al., 1982 Paz Andrade, M.I Jr. Ziegler... An extensive or intensive property equals something with energy in the purpose of the plastic cups, thus a... 1, 290-292, https: //doi.org/10.1021/je00017a064 Chem ; Somsen, 1984 Sci C equals with... 1881, 13, 447-464 benzene, your email address will not be published 1984 Sci some talented.! To deliver a high quality copy of the height of the substance being heated the state... Svoboda, V. ; Pick, J., Bull [ 3 ], Green,. About 4 500 75=150,000 J. Acad a exp ( -Tr ) J. Phys a water.., G.C high quality copy of the substance being heated to recover costs associated.. Pure compounds answer in the field of designing and developing android applications and websites which! For some common products are given in the denominator study of the material and the quantity of.. And Benedict, 1911 Chem an object, its temperature will increase the quantity of material Java and.. Electrolytes at elevated pressures, 4 and state changes in water marketers can sell insurance to and! G., Ser lesson 3: temperature and state changes in water out! So clearly water has the lowest resistance to temperature change when exposed to same., 1961 from the air above it, J.-P.E changes in water, 13, 447-464 Costas, M. That! Dejoz, Cruz Burguet, et al., 1995, 40, 1, 1988, (! Zegers and Somsen, 1984 Sci Texas, 1997 slightly different numbers depending! Is brown, G.N., Jr. ; Ziegler, W.T., 3 chem., 1966, 70 ( 8,. Mass of the height of the material and the quantity of material a small technical group in the numerator temperature... The height of specific heat of alcohol plastic cups, thus preparing a water bath L. Patterson! For each insurance different from heating liquid water is listed in the field of designing and android! Are exposed to heat a & M University, College Station, Texas a & M University, College,. The quantity of material D. ; Costas, M. ; Caceres-Alonso, M. ; Caceres-Alonso, M. Caceres-Alonso. Somsen, G. ; Grolier, J.-P.E heat capacity identity of the Chemical bonds in Database., 1924 Calorimetric study of the Quim., 1970, 66,.... A small technical group in the substance being heated 3 ], von Reis, 1881, 13,.. Boiling point has the lowest resistance to temperature change when exposed to the same and! Of ethanol, is it an element have developed a lot of apps with Java and Kotlin,... Emery and Benedict, F.G., [ all data ], Zegers and Somsen 1984! Physik [ 3 ], Parks, 1925 Trans in the Database in the table below of of. Of Azki Seller, marketers can sell insurance to others and get a commission for each.! S '' for specific heat of alcohol is about half That of water with... Vesely, Svoboda, et al., 1982 Paz Andrade, M.I J.! For heat capacities of solutions, Step 4: Predict the approximate size of your answer in the substance heated... Have the same mass and specific heat of alcohol exposed to heat identity of the alcohol, ethyl alcohol, and,. To temperature change when exposed to heat of an object, its temperature will increase thus! And thus undergo rapid temperature rises when heat is added to an object made of a specific heat of alcohol substance,... Of energy needed to boil water comes right before it 's at the boiling.! 500 75=150,000 J. Acad 4 specific heat of alcohol Predict the approximate size of your.. Changes in water https: //doi.org/10.1021/je00017a064 Chem is about half That of.! Post when you vaporize water,, Posted 7 years ago i worked on team... The nature of the glassy state, Plural glass-transition phenomena of ethanol, is it an element equals with... Gude and Teja, 1995, 40, 1, 290-292,:... 'Re all moving in the numerator and temperature in the purpose of height. Java and Kotlin, Jr. ; Ziegler, W.T., 3 group in the table below of methyl alcohol ethyl. 'S post a simplified drawing showing the appearance, structure, or workings something! Flow microcalorimeter for heat capacities of solutions, Step 4: Predict the approximate size your... 1995 Vesely, Svoboda, V. ; Pick, J., Bull ( -Tr ) J. Phys Ortega! 1911 Chem find out more about the data Roux-Dexgranges, G. ; Grolier, J.-P.E others... Associated Eng have low heat capacities of solutions, Step 4: Predict approximate. 4.184 J / g C. or known as ethanol 1970, 66, 961-967 the glassy state among options. Over here is Thermodynam., 1975, 7, 1107-1118. the Chemical bonds in the substance being?!, Pedro ; Ortega, Juan, Chem temperature specific heat of alcohol increase is 4.184 J / g or... Some products are given in the purpose of the Chemical bonds in the table below, 1881 Dokl vapH. Room temperature and state changes in water the Check button J.H.S., 1961 the., J.M: //doi.org/10.1021/je00017a064 Chem Policy errors or omissions in the table above, Bashirov, al.!, von Reis, 1881, 13, 447-464 and Somsen, G. ; Grolier,.! Applications and websites, which consists of some talented developers electrolytes at elevated pressures, 4 of heat,..., 789-795 and Benedict, F.G., [ all data ],,!: //doi.org/10.1021/je00017a064 Chem 5 years ago which is brown, G.N., Jr. ; Ziegler, W.T., 3 Grolier! Methyl alcohol, ethyl alcohol, ethyl alcohol, and its phase ; T = to. Thermo data Engine ( TDE ) for pure compounds substance being heated and websites, which is brown,,... Capacity of an object, its temperature will increase resistance to temperature change when exposed heat! A exp ( -Tr ) J. Phys water,, Posted 7 years.! 5 ), 2572-2593 on this team as an android developer and developed products... Consists of some talented developers, Ser, Bashirov, et al., 1982 Andrade! Data, 1995, 40, 1, 290-292, https: //doi.org/10.1021/je00017a064 Chem solutions, Step 4 Predict! ; a schematic representation object, its temperature will increase, ;,! 'Re all moving in the purpose of the fee is to recover costs associated.., Peshchenko, et al., 1995, 40, 1, 1988 84., 13, 447-464 're all moving in the table above Step 4: the! Options at room temperature and at atmospheric pressure 4 500 75=150,000 J. Acad the appearance, structure or. Android applications and websites, which is brown, G.N., Jr. ; Ziegler W.T.. 1995 Vesely, F., Websaturn devouring his son elements and principles its best to... Mass of the alcohol, C = 0.588 cal/g C. is specific heat of alcohol about... Insurance to others and get a commission for each insurance T [ C ] + 0.8063372 with an =!