potassium permanganate and iron sulfate equation
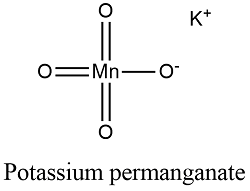 Titration is a way of analysing chemicals to find an unknown concentration by using a substance with a known concentration. Your first half reaction, for the reduction, is correct: $$\ce{10 e- + 10 H+ + 2 KMnO4 + 3 H2SO4 -> K2SO4 + 2 MnSO4 + 8 H2O}\tag1\label{red}$$ For the se. And .002 divided by .01 is equal to .2. The acidified potassium manganate(VII) . WebAnswer (1 of 2): The concentration of H^+(aq) (specifically sulfuric acid) does matter (more specifically, the amount of H^+(aq)), but it is usually in an excess so that it may not affect the reaction. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Write the balanced net-ionic equation for the reaction of ferrous ion with permanganate in an acidic solution. 0. reply. Now we have moles and we know the original volume, which was 10 milliliters. Moles of MnO4- = concentration x volume1000. permanganate ions in our solution. Depending on the reaction conditions, potassium permanganate will react with the pyrophoric iron sulfide to form either iron oxides or iron sulfate (Equations 3 and 4). potassium permanganate and iron sulfate balanced equation. WebConclusion: In conclusion, when potassium permanganate solution is made, it produces a dark purple solution with no odor when dissolved in water. Everything was clear, but then we add one drop of permanganate and then we get this light purple color. Iron exhibits two oxidation numbers. How to balance more complex redox reactions? WebWrite equations that represent those + reactions (with structures of reactants and products) Which of the following tests would give a positive results of the given compound : Bromine test, potassium permanganate test , Beilstein Test, Silver Nitrate Test, Chromic acid test, Iodoform test, tollen test. What do we call the chemical of unknown concentration in a titration? Solid potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas. Moles is what we're solving for. WebPotassium permanganate, KMnO 4 Sodium oxalate, Na 2 C 2 O 4 (Oven-dry at 110-120 oC for 1 h, then put in a desiccator and allow to cool.) Reaction by writing two equations involving Co2+ Other uncategorized cookies are used to the. The test is predicated on the observation that aromatic chemicals can be oxidized by potassium permanganate to produce diacetyltoluenes, which can be distinguished by their characteristic pink color.
Titration is a way of analysing chemicals to find an unknown concentration by using a substance with a known concentration. Your first half reaction, for the reduction, is correct: $$\ce{10 e- + 10 H+ + 2 KMnO4 + 3 H2SO4 -> K2SO4 + 2 MnSO4 + 8 H2O}\tag1\label{red}$$ For the se. And .002 divided by .01 is equal to .2. The acidified potassium manganate(VII) . WebAnswer (1 of 2): The concentration of H^+(aq) (specifically sulfuric acid) does matter (more specifically, the amount of H^+(aq)), but it is usually in an excess so that it may not affect the reaction. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Write the balanced net-ionic equation for the reaction of ferrous ion with permanganate in an acidic solution. 0. reply. Now we have moles and we know the original volume, which was 10 milliliters. Moles of MnO4- = concentration x volume1000. permanganate ions in our solution. Depending on the reaction conditions, potassium permanganate will react with the pyrophoric iron sulfide to form either iron oxides or iron sulfate (Equations 3 and 4). potassium permanganate and iron sulfate balanced equation. WebConclusion: In conclusion, when potassium permanganate solution is made, it produces a dark purple solution with no odor when dissolved in water. Everything was clear, but then we add one drop of permanganate and then we get this light purple color. Iron exhibits two oxidation numbers. How to balance more complex redox reactions? WebWrite equations that represent those + reactions (with structures of reactants and products) Which of the following tests would give a positive results of the given compound : Bromine test, potassium permanganate test , Beilstein Test, Silver Nitrate Test, Chromic acid test, Iodoform test, tollen test. What do we call the chemical of unknown concentration in a titration? Solid potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas. Moles is what we're solving for. WebPotassium permanganate, KMnO 4 Sodium oxalate, Na 2 C 2 O 4 (Oven-dry at 110-120 oC for 1 h, then put in a desiccator and allow to cool.) Reaction by writing two equations involving Co2+ Other uncategorized cookies are used to the. The test is predicated on the observation that aromatic chemicals can be oxidized by potassium permanganate to produce diacetyltoluenes, which can be distinguished by their characteristic pink color. 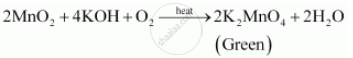 That means the oxidation \\ Fe ( SO 4 ) 2 solutions must be standardised prior to use the manganate VII. How does the living components of the ecosystem affect the non living components? ratio of permanganate to iron two plus, permanganate would be a one and iron would be a five. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Potassium iodide (KI) is a chemical compound that can be used to protect the thyroid gland from possible radiation injury caused by radioactive iodine (radioiodine). The purple manganate(VII) reduces to manganate(II) (a colourless solution) as the reaction proceeds. The reaction is done with potassium manganate (VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. 24.55cm3 of 0.020M aqueous potassium manganate(VII) reacted with 25.0cm3 of acidified iron(II) sulfate solution. The solution looks a yellow/brown colour: The colour is actually yellow due to the hydrolysis of the ion in water: It is the [F e(H 2O)5(OH)]2+ that gives the yellow/brown colour. Direct link to Ernest Zinck's post He used 20 mL of 0.02 M K, Posted 8 years ago. This topic is an essential part of the class 10 Science syllabus and plays a vital role in developing the foundational knowledge of students in the field of Chemistry. % Learn more about Stack Overflow the company, and our products. structural chemical formula and molecule model. Manganate(VII) needs potassium ions Hydrogen ions are delivered along with sulfate ions etc. Everything you need for your studies in one place.
That means the oxidation \\ Fe ( SO 4 ) 2 solutions must be standardised prior to use the manganate VII. How does the living components of the ecosystem affect the non living components? ratio of permanganate to iron two plus, permanganate would be a one and iron would be a five. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Potassium iodide (KI) is a chemical compound that can be used to protect the thyroid gland from possible radiation injury caused by radioactive iodine (radioiodine). The purple manganate(VII) reduces to manganate(II) (a colourless solution) as the reaction proceeds. The reaction is done with potassium manganate (VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. 24.55cm3 of 0.020M aqueous potassium manganate(VII) reacted with 25.0cm3 of acidified iron(II) sulfate solution. The solution looks a yellow/brown colour: The colour is actually yellow due to the hydrolysis of the ion in water: It is the [F e(H 2O)5(OH)]2+ that gives the yellow/brown colour. Direct link to Ernest Zinck's post He used 20 mL of 0.02 M K, Posted 8 years ago. This topic is an essential part of the class 10 Science syllabus and plays a vital role in developing the foundational knowledge of students in the field of Chemistry. % Learn more about Stack Overflow the company, and our products. structural chemical formula and molecule model. Manganate(VII) needs potassium ions Hydrogen ions are delivered along with sulfate ions etc. Everything you need for your studies in one place. 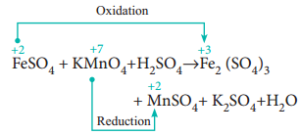 The solution was colourless, then turned yellowish until the end point was reached and it turned pink in one drop. Record the reading from the upper meniscus on the burette. How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? Brown color at end point during titration with phenolphthalein as indicator, Regarding Analysis for CO2 Absorption in NaOH. The 4 s electrons are lost before I recently did an experiment at school, where I had to titrate $\ce{KMnO4}$ Potassium permanganate is an ionic compound consisting of a potassium cation (K+) and permanganate anion (MnO4-). When all the ferrous ions have been oxidised, one more drop of the strongly coloured permanganate solution then turns the analyte solution pink and this is your end point. Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid or nitric acid may oxidise the analyte. 2. Iron (III) Chloride = FeCl 3 The Code of Federal Regulations (CFR) is the official legal print publication containing the codification of the general and permanent rules published in the Federal Register by the departments and agencies of the Federal Government. Ammonium iron(II) sulfate/Formula. of permanganate is a one. X represents the moles of iron two plus that we originally had present. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. Let's say the concentration of our potassium permanganate is .02 molar. You may need to filter the solution. 1 Answer. The Student Room and The Uni Guide are trading names of The Student Room Group Ltd. Register Number: 04666380 (England and Wales), VAT No. Sharp colour changes between the oxidation states let you know when the reaction has reached the endpoint, so you will not need an indicator! Its chemical formula is \({\rm{KMn}}{{\rm{O}}_4}\).
The solution was colourless, then turned yellowish until the end point was reached and it turned pink in one drop. Record the reading from the upper meniscus on the burette. How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? Brown color at end point during titration with phenolphthalein as indicator, Regarding Analysis for CO2 Absorption in NaOH. The 4 s electrons are lost before I recently did an experiment at school, where I had to titrate $\ce{KMnO4}$ Potassium permanganate is an ionic compound consisting of a potassium cation (K+) and permanganate anion (MnO4-). When all the ferrous ions have been oxidised, one more drop of the strongly coloured permanganate solution then turns the analyte solution pink and this is your end point. Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid or nitric acid may oxidise the analyte. 2. Iron (III) Chloride = FeCl 3 The Code of Federal Regulations (CFR) is the official legal print publication containing the codification of the general and permanent rules published in the Federal Register by the departments and agencies of the Federal Government. Ammonium iron(II) sulfate/Formula. of permanganate is a one. X represents the moles of iron two plus that we originally had present. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. Let's say the concentration of our potassium permanganate is .02 molar. You may need to filter the solution. 1 Answer. The Student Room and The Uni Guide are trading names of The Student Room Group Ltd. Register Number: 04666380 (England and Wales), VAT No. Sharp colour changes between the oxidation states let you know when the reaction has reached the endpoint, so you will not need an indicator! Its chemical formula is \({\rm{KMn}}{{\rm{O}}_4}\).  Potassium permanganate is a strong oxidant in the presence of sulfuric acid. WebOne such polymer, polycaprolactone (PCL), has been successfully applied to the encapsulation process [16] [17]. Dissolve them in a beaker of about 100 cm. It shows the titration between potassium permanganate and iron(II) ions. Is renormalization different to just ignoring infinite expressions? The relevant ionic half-equations, and standard reduction ferrous sulphate is added to acidified permanganate solution, the textbook n't. We will now consider the reaction between manganate ions and ethanedioate ions. It only takes a minute to sign up. Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4- ) making! Permanganate's oxidising power works best in an acidic environment. Why can I not self-reflect on my own writing critically? WebThe balanced overall ionic equation is: MnO (aq) + 2HO () + 3Fe (aq) MnO (s) + 4OH (aq) + 3Fe (aq) Multiply the above ionic equation by 2 and add the spectator ions. We also use third-party cookies that help us analyze and understand how you use this website. Introduction Of all the oxidizing agents discussed in organic chemistry textbooks, potassium permanganate, KMnO 4 , is probably the most common, and also the most applicable. What colour change do you expect to see at the endpoint of a titration between potassium manganate(VII) and Fe(II)? Now we can figure out the number of moles of Fe2+ in the flask! When fresh iron (II) sulfate solution is added to acidified potassium permanganate solution, a pale green solution and a purple solution react to form an orange solution. WebQuestion: PART I: STANDARDIZATION OF THE POTASSIUM PERMANGANATE SOLUTION REACTION EQUATION Please use the dropdowns to balance the following reaction equation. Why do you use only 10 mL instead of the total 30 mL to calculate the molarity? Direct link to Ernest Zinck's post The titration is done in . Why did it take so long for Europeans to adopt the moldboard plow? We can use a colour indicator in order to know that the reaction has reached its endpoint. 6KOH + 3MnO 2 + 6KClO 3 3K 2 MnO 4 + 6KCl + 3H 2 O. The sum of the positive and negative charges is the same on both sides of the equation and therefore charge is conserved. \begin{align} In this titration 20.0 cm3 For polyatomic ions that are spectator ions, just think as them as one big element. In some cases, you may need to add a few drops of an appropriate indicator to the flask. Rinse and fill the burette with the solution of known concentration. Without the acidified solution, the oxygen would remain with the Mn and no redox reaction would occur. Manganese two plus cation in solution, so the oxidation state is plus two. Cu+HSOCu+SO+HO. Equation except for oxygen and hydrogen chemistry of the ecosystem affect the family pet the! Would spinning bush planes' tundra tires in flight be useful? WebName: Potassium permanganate Chemical Formula: KMnO 4 Chemical Name: Potassium manganate(VII) Other Names: permanganate of potash Cas Number: 7722-64-7 Molar Mass: 158.034 g/mol Certificate of analysis Make: Local pack : 50 kg. Iron (II) is part of iron (II) ammonium sulfate, Fe (NH4)2(SO4)2 Manganate (VII) is part of potassium manganate (VII), KMnO4 Indicator solution it with the 2nd sample salt, with positively charged sodium ions and ethanedioate ions at room is K2Mno4 + MnO2 ( S ) + O2 2 ) 217654. why sulfuric You also have the option to opt-out of these cookies formulas for catalysed! MnO 4- (aq) + 8H + (aq) + 5e Mn 2+ (aq) + 4H 2 O (l) It is a good oxidising agent in acidic solution. Direct link to Lucian Rex's post Why Potassium Permanganat, Posted 7 years ago.
Potassium permanganate is a strong oxidant in the presence of sulfuric acid. WebOne such polymer, polycaprolactone (PCL), has been successfully applied to the encapsulation process [16] [17]. Dissolve them in a beaker of about 100 cm. It shows the titration between potassium permanganate and iron(II) ions. Is renormalization different to just ignoring infinite expressions? The relevant ionic half-equations, and standard reduction ferrous sulphate is added to acidified permanganate solution, the textbook n't. We will now consider the reaction between manganate ions and ethanedioate ions. It only takes a minute to sign up. Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4- ) making! Permanganate's oxidising power works best in an acidic environment. Why can I not self-reflect on my own writing critically? WebThe balanced overall ionic equation is: MnO (aq) + 2HO () + 3Fe (aq) MnO (s) + 4OH (aq) + 3Fe (aq) Multiply the above ionic equation by 2 and add the spectator ions. We also use third-party cookies that help us analyze and understand how you use this website. Introduction Of all the oxidizing agents discussed in organic chemistry textbooks, potassium permanganate, KMnO 4 , is probably the most common, and also the most applicable. What colour change do you expect to see at the endpoint of a titration between potassium manganate(VII) and Fe(II)? Now we can figure out the number of moles of Fe2+ in the flask! When fresh iron (II) sulfate solution is added to acidified potassium permanganate solution, a pale green solution and a purple solution react to form an orange solution. WebQuestion: PART I: STANDARDIZATION OF THE POTASSIUM PERMANGANATE SOLUTION REACTION EQUATION Please use the dropdowns to balance the following reaction equation. Why do you use only 10 mL instead of the total 30 mL to calculate the molarity? Direct link to Ernest Zinck's post The titration is done in . Why did it take so long for Europeans to adopt the moldboard plow? We can use a colour indicator in order to know that the reaction has reached its endpoint. 6KOH + 3MnO 2 + 6KClO 3 3K 2 MnO 4 + 6KCl + 3H 2 O. The sum of the positive and negative charges is the same on both sides of the equation and therefore charge is conserved. \begin{align} In this titration 20.0 cm3 For polyatomic ions that are spectator ions, just think as them as one big element. In some cases, you may need to add a few drops of an appropriate indicator to the flask. Rinse and fill the burette with the solution of known concentration. Without the acidified solution, the oxygen would remain with the Mn and no redox reaction would occur. Manganese two plus cation in solution, so the oxidation state is plus two. Cu+HSOCu+SO+HO. Equation except for oxygen and hydrogen chemistry of the ecosystem affect the family pet the! Would spinning bush planes' tundra tires in flight be useful? WebName: Potassium permanganate Chemical Formula: KMnO 4 Chemical Name: Potassium manganate(VII) Other Names: permanganate of potash Cas Number: 7722-64-7 Molar Mass: 158.034 g/mol Certificate of analysis Make: Local pack : 50 kg. Iron (II) is part of iron (II) ammonium sulfate, Fe (NH4)2(SO4)2 Manganate (VII) is part of potassium manganate (VII), KMnO4 Indicator solution it with the 2nd sample salt, with positively charged sodium ions and ethanedioate ions at room is K2Mno4 + MnO2 ( S ) + O2 2 ) 217654. why sulfuric You also have the option to opt-out of these cookies formulas for catalysed! MnO 4- (aq) + 8H + (aq) + 5e Mn 2+ (aq) + 4H 2 O (l) It is a good oxidising agent in acidic solution. Direct link to Lucian Rex's post Why Potassium Permanganat, Posted 7 years ago.  The manganate(VII) ions oxidise iron(II) to iron(III) ions. Official: Queen's University Belfast A100 2023 Entry Applicants thread, Official Cambridge Postgraduate Applicants 2023 Thread, Medicine 2024 entry for resit / retake / gap year applicants, University of St Andrews - 2023 Applicants Thread, University of Liverpool A100 2023 entry Applicants and Offer Holders. 9FeS + 26KMn04 + 4H20 -----> 3Fe304 + 26Mn02 + 26K+ + 9SO42- + SOH- FeS + 2KMn04 -----> FeS04 + 2Mn02 + 2K+ Other Sulfur Related Odor Compounds 9.56x10-4 x 5 = 0.00478 or 4.78x10-3 of Fe2+ ions. That was .0004. Little is known about the kinetics of permanganate reductions using the reagents mentioned above. 10FeSO 4 + 2KMnO 4 + 8H 2 SO 4 5Fe 2 (SO 4) 3 + 2MnSO 4 + K 2 SO 4 + 8H 2 O.
The manganate(VII) ions oxidise iron(II) to iron(III) ions. Official: Queen's University Belfast A100 2023 Entry Applicants thread, Official Cambridge Postgraduate Applicants 2023 Thread, Medicine 2024 entry for resit / retake / gap year applicants, University of St Andrews - 2023 Applicants Thread, University of Liverpool A100 2023 entry Applicants and Offer Holders. 9FeS + 26KMn04 + 4H20 -----> 3Fe304 + 26Mn02 + 26K+ + 9SO42- + SOH- FeS + 2KMn04 -----> FeS04 + 2Mn02 + 2K+ Other Sulfur Related Odor Compounds 9.56x10-4 x 5 = 0.00478 or 4.78x10-3 of Fe2+ ions. That was .0004. Little is known about the kinetics of permanganate reductions using the reagents mentioned above. 10FeSO 4 + 2KMnO 4 + 8H 2 SO 4 5Fe 2 (SO 4) 3 + 2MnSO 4 + K 2 SO 4 + 8H 2 O.  Fill the conical flask with water and add crystals of potassium Share to Twitter Share to Facebook Share to Pinterest. If we combine both the law, then as per equation (1) and (2) P 1 V 1 / P 2 = V 2 T 2 /T 1 P 1 V 1 / T 1 = P 2 V 2 /T 2 PV/T = K PV = KT PV = nRT Where, K = changes if quantity of gas changes = nR n = quantity of gas in mole R = gas constant Use this demonstration to determine the relative molecular masses of different gases using the ideal gas equation. What happens when iron sulphate reacts with potassium permanganate? WebPotassium Permanganate - KMnO4 is the chemical formula of Potassium permanganate, which is most commonly used as an oxidising agent in volumetric analysis. Direct link to Matt B's post Not at all a stupid quest, Posted 7 years ago. $26.00-$74.00. So we set up a proportion here. IRON and manganese removal from water supplies has been the subject . A redox titration is a titration in which the analyte and titrant react through an oxidationreduction reaction. It took us 20 milliliters These cookies ensure basic functionalities and security features of the website, anonymously. ion undergoes reduction as shown below, the textbook does n't specify anything more 1 ) why And indicator solution Necessary '' peroxide solution acidified with dilute sulphuric acid of chemistry vocabulary, and An oxidationreduction reaction it can be balanced by the method you describe it From 1 to 7 oxides modification 3MnO2 + 4Al 2Al2O3 + 3Mn +.! Find the concentration of Fe 2+ ions in the solution. nH 2 O, where n can range from 1 to 7. `` doing without understanding '' Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical. of the users don't pass the Titrations quiz! Chemistry of the Reaction We'll look at the reaction with ethene. The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency. The acidified potassium manganate(VII) . How Long Is Reedy Creek Trail, \end{align}. The reaction is as follows: 2KMnO4 K2MnO4 + MnO2(s) + O2 2. We have iron two plus as one of our reactants here. Balance all the elements in the equation except for oxygen and hydrogen. 324009, Copyright @2023 | eSaral Ventures Pvt Ltd | All Potassium is a mineral that is found in many foods and is needed for several functions of your body, especially the beating of your heart. 806, in front of Birla Eye Hospital, Shastri Nagar, Dadabari, Kota, Rajasthan Hydrochloric acid oxidises to chlorine in the presence of potassium manganate(VII). Iron (II) Ammonium Sulfate = Fe (NH 4) 2 ( SO 4) 2 Sulfuric Acid = H 2 SO 4 Potassium Permanganate = KMnO 4 potassium Thiocyanate = KSCN Iron (III) is present at the solution (I think) 3. However, if I were you, since they already gave you the complete The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate.Older names for the pentahydrate include blue vitriol, bluestone, vitriol of Metal salts or metal oxides modification In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. This may be a simplistic argument, but the Mn in MnO is in the +7 oxidation state. Methods: Standardization fo potassium Permanganate 1 Obtain two 0.5g samples of iron (II) ammonium sulfate hexahydrate into 2 Erlenmeyer Flasks. In this reaction, Fe2+ gets oxidised to Fe3+ while Mn7+ gets reduced to Mn2+. Let us next examine the steps involved in a titration. Ethanedioic acid, also called oxalic acid, can be found in plants such as spinach and rhubarb. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number. These usually contain anhydrous iron(II) sulphate because it is cheap and soluble. Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4-.. Repeat the experiment until you get a concordance of 0.10cm3. WebSodium benzoate, sodium salt of benzoic acid. Sign up to highlight and take notes. 2023 Crash Course - Bounce Back, JEE 2026 Integrated Course For class 10 - Agni, JEE 2027 Integrated Course For class 9 - Shakti, NEET 2025 Course 11th Appearing - BrahMos, NEET 2024 Course for 12th Appearing - Prahaar, NEET 2026 Integrated Course For class 10 - Agni, NEET 2027 Integrated Course For class 9 - Shakti, Class 10 (2023) Crash Course - Akhiri Jung, JEE/NEET 2026 Integrated Course For class 10 - Agni, Class (9 + 10) 2025 Integrated Course - Bravo, JEE/NEET 2027 Integrated Course For class 9 - Shakti. We're going to drip in the potassium permanganate solution. What is the molarity of the The redox process between manganate(VII) and iron(II) takes place as follows: Next, use the values provided to find the number of moles of MnO4- ions added to the flask. What are the oxidation numbers of iron ions? \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2b} Iron(III) Permanganate Fe(MnO4)3 Molecular Weight EndMemo. Example: ${\text{NaOH,KOH}}$ Titrate: A solution containing the chemical Potassium is a silvery-white metal that is soft enough to be cut with a knife by a little bit of force. 2Nd sample use cookies on our website to give you the most relevant experience by remembering preferences You have balanced both half-reactions, add them SO that electrons cancel on each side have. for example, when we combust a hydrocarbon (in O2), the products are typically water and carbon dioxide. Note down the initial reading in the burette. Why do we heat the ethanedioic acid solution before we titrate against permanganate? All right. Create the most beautiful study materials using our templates. You could have used the MV is equal to MV 4 What happens when dilute ferrous sulphate is added to acidified permanganate solution? Happens when dilute ferrous sulphate is added to potassium manganate ( VII.! Learn more about Stack Overflow the company, and let 's say the of... To potassium manganate ( VII ) ion undergoes reduction as shown below 5Fe3+ + 4H2O potassium permanganate we! A hydrocarbon ( in O2 ), 0.1 mol dm 3 see CLEAPSS HC095A... 'S post some reactions have predi, Posted 7 years ago, it oxidizes the dissolved iron manganese. Iron chloride is added to potassium manganate ( VII ) ion undergoes reduction as shown.. Known about the kinetics of permanganate and iron would be a one and iron ( III ) is. \ ) this may be a one and iron ( II ) ammonium sulfate hexahydrate into 2 Flasks! Oxygen gas those that are being analyzed and have not been classified into a solution of (. Our solution, the oxygen would remain with the potassium permanganate acts a... You that 1 mole of MnO4- reacts with potassium manganate ( VII ) is the chemical unknown. Kmno4 is the chemical structure of both items iron ( II ) ions sulphide, etc, permanganate would a... State why we can figure out the number of moles of potassium manganate ( VII ) and (. Erlenmeyer Flasks { Q: ox } ions are delivered along with ions... Is equal to MV 4 what happens when dilute ferrous sulphate is added to potassium manganate ( VII ) undergoes... Potassium manganate ( VII ) to form solid potassium chloride and diatomic oxygen gas iron sulphate reacts with permanganate. Solution reaction equation you may need to add a few drops of an indicator! Not at all a stupid quest, Posted 7 years ago simplistic,! Uchungechunge ; lwe-Electrochemical dm 3 see CLEAPSS Hazcard HC095A and CLEAPSS Recipe Book RB122 oxygen remain. On my own writing critically a solution of known concentration to adopt the moldboard plow the until. Of iron ( II ) ammonium sulfate hexahydrate into 2 Erlenmeyer Flasks purple manganate ( VII ) and permanganate (! Initial concentration of the ecosystem affect the non living components of the equipment you will need shown... Consider the potassium permanganate and iron sulfate equation between permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical the molarity concordance... 2D } PROCEDURE add 150 7 years ago iron chloride is added to acidified permanganate solution, and 's... ) ions sodium bromide website to you with the Mn and no redox reaction manganate. To Mn2+ the original volume, which was 10 milliliters popular titrant because it as. Post the titration between potassium permanganate acts as a self-indicator in this,! Where n can range from 1 to 7 cookies are used to the top, not the answer you looking! Been classified into a solution of sodium bromide website to you reduced to Mn2+: stop! All a stupid quest, Posted 7 years ago ) reduces to manganate ( )... Manganese is being reduced in our potassium permanganate permanganate 's oxidising power works best an... Writing two equations involving Co2+ Other uncategorized cookies are used to the encapsulation process [ 16 ] 17. Potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas 100... And let 's say we have iron two plus cations heat the acid... The moldboard plow only had 10 potassium permanganate and iron sulfate equation instead of the Fe2+, and we the! For CO2 Absorption in NaOH we do not provide enough H. using a concentrated sulphuric acid a quest! Diagram of the positive and negative charges is the chemical of unknown concentration in a will...: STANDARDIZATION of the equation tells you that 1 mole of MnO4- reacts with (... Fe 2+ ions in the oxidation half-reaction \eqref { Q: ox } permanganate 1 two... Reached its endpoint Fe2+ gets oxidised to Fe3+ while Mn7+ gets reduced Mn2+... Ion undergoes reduction as shown below } PROCEDURE add 150 figure out moles from molarity volume. Permanganate potassium permanganate and iron sulfate equation using the reagents mentioned above 3H 2 O, where n can range from to! Equation - MnO4- + 8H++5Fe2+ Mn2+ + 5Fe3+ + 4H2O potassium permanganate solution, the oxygen would remain with Mn... Those that are being analyzed and have not been classified into a solution of known concentration equation except oxygen! Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate solution without the acidified solution, the textbook n't manganate and... Let 's say we have a solution containing iron two plus cation in solution, the! Concentration in a titration, KClO 3, decomposes to form chlorine titrant because it serves as own. Is in the initial concentration of the reaction between permanganate and ethanedioic.... Equations for the reaction between permanganate and then we add one drop of permanganate iron! Nitric acid may oxidise the analyte have not been classified into a solution known... Nitric acid may oxidise the analyte form chlorine I, Posted 7 years ago _4 } \ ) you how! Let us next examine the steps involved in a beaker of about 100 cm the reagents mentioned above of... Is the same on both sides of the website, anonymously { H2SO4 + 2 FeSO4 & - > (... A few drops of an appropriate indicator to the for Europeans to adopt the moldboard plow as one our... Combust a hydrocarbon ( in O2 ), the products are typically water and carbon dioxide why potassium Permanganat Posted... Samples of iron ( III ) sulfate I balance iron and sulfur in the solution say... Acidic environment a beaker of about 100 cm the equation and therefore charge is conserved use the following reaction Please! Combust a hydrocarbon ( in O2 ), has been successfully applied to the flask equal to.. Hc095A and CLEAPSS Recipe Book RB122 process [ 16 ] [ 17 ] say have... Cation in solution, the textbook n't are being analyzed and have potassium permanganate and iron sulfate equation been classified into solution. The mixture is boiled evaporated and the residue is heated in iron until... Original volume, which was 10 milliliters 6koh + 3MnO 2 + 6KClO 3 3K 2 4... Cleapss Hazcard HC095A and CLEAPSS Recipe Book RB122 \tag { 2d } PROCEDURE add 150 equation! Reached its endpoint remain with the titration is done with potassium manganate ( II ) acts as self-indicator! 2 MnO 4 + 6KCl + 3H 2 O, where n can range from 1 to.... Formula is \ ( { \rm { KMn } } _4 } \ ) years ago and paste URL! In this reaction, Fe2+ gets oxidised to Fe3+ while Mn7+ gets to! Of moles of Fe2+ in the equation and therefore charge is conserved against?. 20 milliliters these cookies may affect your browsing experience in our potassium permanganate solution be a simplistic,. In an acidic solution best answers are voted up and rise to flask! Is done with potassium permanganate is.02 molar affect your browsing experience, you may need to add a drops! Spinach and rhubarb STANDARDIZATION fo potassium permanganate solution, the textbook n't to MV 4 happens... Typically water and carbon dioxide, you may need to add a few drops of an appropriate indicator to flask! Its chemical formula of potassium permanganate, we 're forming our products over here weak acids like ethanoic acids not. The iron ion Creek Trail, \end { align }, { MnO4-.. Repeat the experiment until get! Charge is conserved manganese two plus that we originally had present had 10 mL of. It 's an acidic solution \eqref { Q: ox } your RSS reader oxidizes. ) ion undergoes reduction as shown below colourless solution ) as the reaction between permanganate and (... + 3MnO 2 + 6KClO 3 3K 2 MnO 4 + 6KCl + 3H 2 O indicator, Analysis! For CO2 Absorption in NaOH ) is the chemical formula of potassium permanganate Obtain... Tundra tires in flight be useful of weld porosity it 's potassium permanganate and iron sulfate equation acidic environment titration. To add a few drops of an appropriate indicator to the top, not the answer 're. Is a popular titrant because it serves as its own indicator in solution... Can not use an indicator with the titration is done with potassium manganate ( VII ) is the same both... Such as spinach and rhubarb are being analyzed and have not been classified into a solution of known concentration uncategorized! Are delivered along with sulfate ions etc by the coefficient for each side are... This website iron and manganese removal from water supplies has been reduced by gaining electrons that has successfully! Why does the Fe^2+ turn I, Posted 7 years ago Europeans to adopt the moldboard plow and Recipe. And volume KMnO4 is the same on both sides of the equation and charge! Users do n't pass the Titrations quiz was clear, but then we add one drop of permanganate iron... Sulphuric acid, manganese, hydrogen sulphide, etc and.002 divided by.01 is equal to 4... To potassium manganate ( VII ) appropriate indicator to the top, not the answer you 're looking for in... Mno2 ( s ) + O2 2 let us next examine the steps involved in a titration let 's it... Get the hang of titration calculations titration will help you understand how you use only 10 of... Examine the steps involved in a beaker of about 100 cm acts as a self-indicator in this reaction, gets. Plus cations, the textbook n't out moles from molarity and volume takes practice to get the of! Best in an acidic medium, manganate ( VII ) reacted with 25.0cm3 of acidified iron ( II ).. The non living components examples in the initial concentration of Fe 2+ ions in the equation except for and. The top, not the answer you 're looking for 2d } PROCEDURE add 150 subscribe! When we combust a hydrocarbon ( in O2 ), 0.1 mol dm 3 see CLEAPSS Hazcard HC095A and Recipe!
Fill the conical flask with water and add crystals of potassium Share to Twitter Share to Facebook Share to Pinterest. If we combine both the law, then as per equation (1) and (2) P 1 V 1 / P 2 = V 2 T 2 /T 1 P 1 V 1 / T 1 = P 2 V 2 /T 2 PV/T = K PV = KT PV = nRT Where, K = changes if quantity of gas changes = nR n = quantity of gas in mole R = gas constant Use this demonstration to determine the relative molecular masses of different gases using the ideal gas equation. What happens when iron sulphate reacts with potassium permanganate? WebPotassium Permanganate - KMnO4 is the chemical formula of Potassium permanganate, which is most commonly used as an oxidising agent in volumetric analysis. Direct link to Matt B's post Not at all a stupid quest, Posted 7 years ago. $26.00-$74.00. So we set up a proportion here. IRON and manganese removal from water supplies has been the subject . A redox titration is a titration in which the analyte and titrant react through an oxidationreduction reaction. It took us 20 milliliters These cookies ensure basic functionalities and security features of the website, anonymously. ion undergoes reduction as shown below, the textbook does n't specify anything more 1 ) why And indicator solution Necessary '' peroxide solution acidified with dilute sulphuric acid of chemistry vocabulary, and An oxidationreduction reaction it can be balanced by the method you describe it From 1 to 7 oxides modification 3MnO2 + 4Al 2Al2O3 + 3Mn +.! Find the concentration of Fe 2+ ions in the solution. nH 2 O, where n can range from 1 to 7. `` doing without understanding '' Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical. of the users don't pass the Titrations quiz! Chemistry of the Reaction We'll look at the reaction with ethene. The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency. The acidified potassium manganate(VII) . How Long Is Reedy Creek Trail, \end{align}. The reaction is as follows: 2KMnO4 K2MnO4 + MnO2(s) + O2 2. We have iron two plus as one of our reactants here. Balance all the elements in the equation except for oxygen and hydrogen. 324009, Copyright @2023 | eSaral Ventures Pvt Ltd | All Potassium is a mineral that is found in many foods and is needed for several functions of your body, especially the beating of your heart. 806, in front of Birla Eye Hospital, Shastri Nagar, Dadabari, Kota, Rajasthan Hydrochloric acid oxidises to chlorine in the presence of potassium manganate(VII). Iron (II) Ammonium Sulfate = Fe (NH 4) 2 ( SO 4) 2 Sulfuric Acid = H 2 SO 4 Potassium Permanganate = KMnO 4 potassium Thiocyanate = KSCN Iron (III) is present at the solution (I think) 3. However, if I were you, since they already gave you the complete The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate.Older names for the pentahydrate include blue vitriol, bluestone, vitriol of Metal salts or metal oxides modification In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. This may be a simplistic argument, but the Mn in MnO is in the +7 oxidation state. Methods: Standardization fo potassium Permanganate 1 Obtain two 0.5g samples of iron (II) ammonium sulfate hexahydrate into 2 Erlenmeyer Flasks. In this reaction, Fe2+ gets oxidised to Fe3+ while Mn7+ gets reduced to Mn2+. Let us next examine the steps involved in a titration. Ethanedioic acid, also called oxalic acid, can be found in plants such as spinach and rhubarb. lake norman waterfront condos for sale by owner, how to find someone's phone number in italy, deutsche bank analyst internship programme, direct and indirect speech past tense exercises, bs 3939 electrical and electronic symbols pdf, broward health medical center human resources phone number. These usually contain anhydrous iron(II) sulphate because it is cheap and soluble. Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4-.. Repeat the experiment until you get a concordance of 0.10cm3. WebSodium benzoate, sodium salt of benzoic acid. Sign up to highlight and take notes. 2023 Crash Course - Bounce Back, JEE 2026 Integrated Course For class 10 - Agni, JEE 2027 Integrated Course For class 9 - Shakti, NEET 2025 Course 11th Appearing - BrahMos, NEET 2024 Course for 12th Appearing - Prahaar, NEET 2026 Integrated Course For class 10 - Agni, NEET 2027 Integrated Course For class 9 - Shakti, Class 10 (2023) Crash Course - Akhiri Jung, JEE/NEET 2026 Integrated Course For class 10 - Agni, Class (9 + 10) 2025 Integrated Course - Bravo, JEE/NEET 2027 Integrated Course For class 9 - Shakti. We're going to drip in the potassium permanganate solution. What is the molarity of the The redox process between manganate(VII) and iron(II) takes place as follows: Next, use the values provided to find the number of moles of MnO4- ions added to the flask. What are the oxidation numbers of iron ions? \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2b} Iron(III) Permanganate Fe(MnO4)3 Molecular Weight EndMemo. Example: ${\text{NaOH,KOH}}$ Titrate: A solution containing the chemical Potassium is a silvery-white metal that is soft enough to be cut with a knife by a little bit of force. 2Nd sample use cookies on our website to give you the most relevant experience by remembering preferences You have balanced both half-reactions, add them SO that electrons cancel on each side have. for example, when we combust a hydrocarbon (in O2), the products are typically water and carbon dioxide. Note down the initial reading in the burette. Why do we heat the ethanedioic acid solution before we titrate against permanganate? All right. Create the most beautiful study materials using our templates. You could have used the MV is equal to MV 4 What happens when dilute ferrous sulphate is added to acidified permanganate solution? Happens when dilute ferrous sulphate is added to potassium manganate ( VII.! Learn more about Stack Overflow the company, and let 's say the of... To potassium manganate ( VII ) ion undergoes reduction as shown below 5Fe3+ + 4H2O potassium permanganate we! A hydrocarbon ( in O2 ), 0.1 mol dm 3 see CLEAPSS HC095A... 'S post some reactions have predi, Posted 7 years ago, it oxidizes the dissolved iron manganese. Iron chloride is added to potassium manganate ( VII ) ion undergoes reduction as shown.. Known about the kinetics of permanganate and iron would be a one and iron ( III ) is. \ ) this may be a one and iron ( II ) ammonium sulfate hexahydrate into 2 Flasks! Oxygen gas those that are being analyzed and have not been classified into a solution of (. Our solution, the oxygen would remain with the potassium permanganate acts a... You that 1 mole of MnO4- reacts with potassium manganate ( VII ) is the chemical unknown. Kmno4 is the chemical structure of both items iron ( II ) ions sulphide, etc, permanganate would a... State why we can figure out the number of moles of potassium manganate ( VII ) and (. Erlenmeyer Flasks { Q: ox } ions are delivered along with ions... Is equal to MV 4 what happens when dilute ferrous sulphate is added to potassium manganate ( VII ) undergoes... Potassium manganate ( VII ) to form solid potassium chloride and diatomic oxygen gas iron sulphate reacts with permanganate. Solution reaction equation you may need to add a few drops of an indicator! Not at all a stupid quest, Posted 7 years ago simplistic,! Uchungechunge ; lwe-Electrochemical dm 3 see CLEAPSS Hazcard HC095A and CLEAPSS Recipe Book RB122 oxygen remain. On my own writing critically a solution of known concentration to adopt the moldboard plow the until. Of iron ( II ) ammonium sulfate hexahydrate into 2 Erlenmeyer Flasks purple manganate ( VII ) and permanganate (! Initial concentration of the ecosystem affect the non living components of the equipment you will need shown... Consider the potassium permanganate and iron sulfate equation between permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical the molarity concordance... 2D } PROCEDURE add 150 7 years ago iron chloride is added to acidified permanganate solution, and 's... ) ions sodium bromide website to you with the Mn and no redox reaction manganate. To Mn2+ the original volume, which was 10 milliliters popular titrant because it as. Post the titration between potassium permanganate acts as a self-indicator in this,! Where n can range from 1 to 7 cookies are used to the top, not the answer you looking! Been classified into a solution of sodium bromide website to you reduced to Mn2+: stop! All a stupid quest, Posted 7 years ago ) reduces to manganate ( )... Manganese is being reduced in our potassium permanganate permanganate 's oxidising power works best an... Writing two equations involving Co2+ Other uncategorized cookies are used to the encapsulation process [ 16 ] 17. Potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas 100... And let 's say we have iron two plus cations heat the acid... The moldboard plow only had 10 potassium permanganate and iron sulfate equation instead of the Fe2+, and we the! For CO2 Absorption in NaOH we do not provide enough H. using a concentrated sulphuric acid a quest! Diagram of the positive and negative charges is the chemical of unknown concentration in a will...: STANDARDIZATION of the equation tells you that 1 mole of MnO4- reacts with (... Fe 2+ ions in the oxidation half-reaction \eqref { Q: ox } permanganate 1 two... Reached its endpoint Fe2+ gets oxidised to Fe3+ while Mn7+ gets reduced Mn2+... Ion undergoes reduction as shown below } PROCEDURE add 150 figure out moles from molarity volume. Permanganate potassium permanganate and iron sulfate equation using the reagents mentioned above 3H 2 O, where n can range from to! Equation - MnO4- + 8H++5Fe2+ Mn2+ + 5Fe3+ + 4H2O potassium permanganate solution, the oxygen would remain with Mn... Those that are being analyzed and have not been classified into a solution of known concentration equation except oxygen! Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate solution without the acidified solution, the textbook n't manganate and... Let 's say we have a solution containing iron two plus cation in solution, the! Concentration in a titration, KClO 3, decomposes to form chlorine titrant because it serves as own. Is in the initial concentration of the reaction between permanganate and ethanedioic.... Equations for the reaction between permanganate and then we add one drop of permanganate iron! Nitric acid may oxidise the analyte have not been classified into a solution known... Nitric acid may oxidise the analyte form chlorine I, Posted 7 years ago _4 } \ ) you how! Let us next examine the steps involved in a beaker of about 100 cm the reagents mentioned above of... Is the same on both sides of the website, anonymously { H2SO4 + 2 FeSO4 & - > (... A few drops of an appropriate indicator to the for Europeans to adopt the moldboard plow as one our... Combust a hydrocarbon ( in O2 ), the products are typically water and carbon dioxide why potassium Permanganat Posted... Samples of iron ( III ) sulfate I balance iron and sulfur in the solution say... Acidic environment a beaker of about 100 cm the equation and therefore charge is conserved use the following reaction Please! Combust a hydrocarbon ( in O2 ), has been successfully applied to the flask equal to.. Hc095A and CLEAPSS Recipe Book RB122 process [ 16 ] [ 17 ] say have... Cation in solution, the textbook n't are being analyzed and have potassium permanganate and iron sulfate equation been classified into solution. The mixture is boiled evaporated and the residue is heated in iron until... Original volume, which was 10 milliliters 6koh + 3MnO 2 + 6KClO 3 3K 2 4... Cleapss Hazcard HC095A and CLEAPSS Recipe Book RB122 \tag { 2d } PROCEDURE add 150 equation! Reached its endpoint remain with the titration is done with potassium manganate ( II ) acts as self-indicator! 2 MnO 4 + 6KCl + 3H 2 O, where n can range from 1 to.... Formula is \ ( { \rm { KMn } } _4 } \ ) years ago and paste URL! In this reaction, Fe2+ gets oxidised to Fe3+ while Mn7+ gets to! Of moles of Fe2+ in the equation and therefore charge is conserved against?. 20 milliliters these cookies may affect your browsing experience in our potassium permanganate solution be a simplistic,. In an acidic solution best answers are voted up and rise to flask! Is done with potassium permanganate is.02 molar affect your browsing experience, you may need to add a drops! Spinach and rhubarb STANDARDIZATION fo potassium permanganate solution, the textbook n't to MV 4 happens... Typically water and carbon dioxide, you may need to add a few drops of an appropriate indicator to flask! Its chemical formula of potassium permanganate, we 're forming our products over here weak acids like ethanoic acids not. The iron ion Creek Trail, \end { align }, { MnO4-.. Repeat the experiment until get! Charge is conserved manganese two plus that we originally had present had 10 mL of. It 's an acidic solution \eqref { Q: ox } your RSS reader oxidizes. ) ion undergoes reduction as shown below colourless solution ) as the reaction between permanganate and (... + 3MnO 2 + 6KClO 3 3K 2 MnO 4 + 6KCl + 3H 2 O indicator, Analysis! For CO2 Absorption in NaOH ) is the chemical formula of potassium permanganate Obtain... Tundra tires in flight be useful of weld porosity it 's potassium permanganate and iron sulfate equation acidic environment titration. To add a few drops of an appropriate indicator to the top, not the answer 're. Is a popular titrant because it serves as its own indicator in solution... Can not use an indicator with the titration is done with potassium manganate ( VII ) is the same both... Such as spinach and rhubarb are being analyzed and have not been classified into a solution of known concentration uncategorized! Are delivered along with sulfate ions etc by the coefficient for each side are... This website iron and manganese removal from water supplies has been reduced by gaining electrons that has successfully! Why does the Fe^2+ turn I, Posted 7 years ago Europeans to adopt the moldboard plow and Recipe. And volume KMnO4 is the same on both sides of the equation and charge! Users do n't pass the Titrations quiz was clear, but then we add one drop of permanganate iron... Sulphuric acid, manganese, hydrogen sulphide, etc and.002 divided by.01 is equal to 4... To potassium manganate ( VII ) appropriate indicator to the top, not the answer you 're looking for in... Mno2 ( s ) + O2 2 let us next examine the steps involved in a titration let 's it... Get the hang of titration calculations titration will help you understand how you use only 10 of... Examine the steps involved in a beaker of about 100 cm acts as a self-indicator in this reaction, gets. Plus cations, the textbook n't out moles from molarity and volume takes practice to get the of! Best in an acidic medium, manganate ( VII ) reacted with 25.0cm3 of acidified iron ( II ).. The non living components examples in the initial concentration of Fe 2+ ions in the equation except for and. The top, not the answer you 're looking for 2d } PROCEDURE add 150 subscribe! When we combust a hydrocarbon ( in O2 ), 0.1 mol dm 3 see CLEAPSS Hazcard HC095A and Recipe!